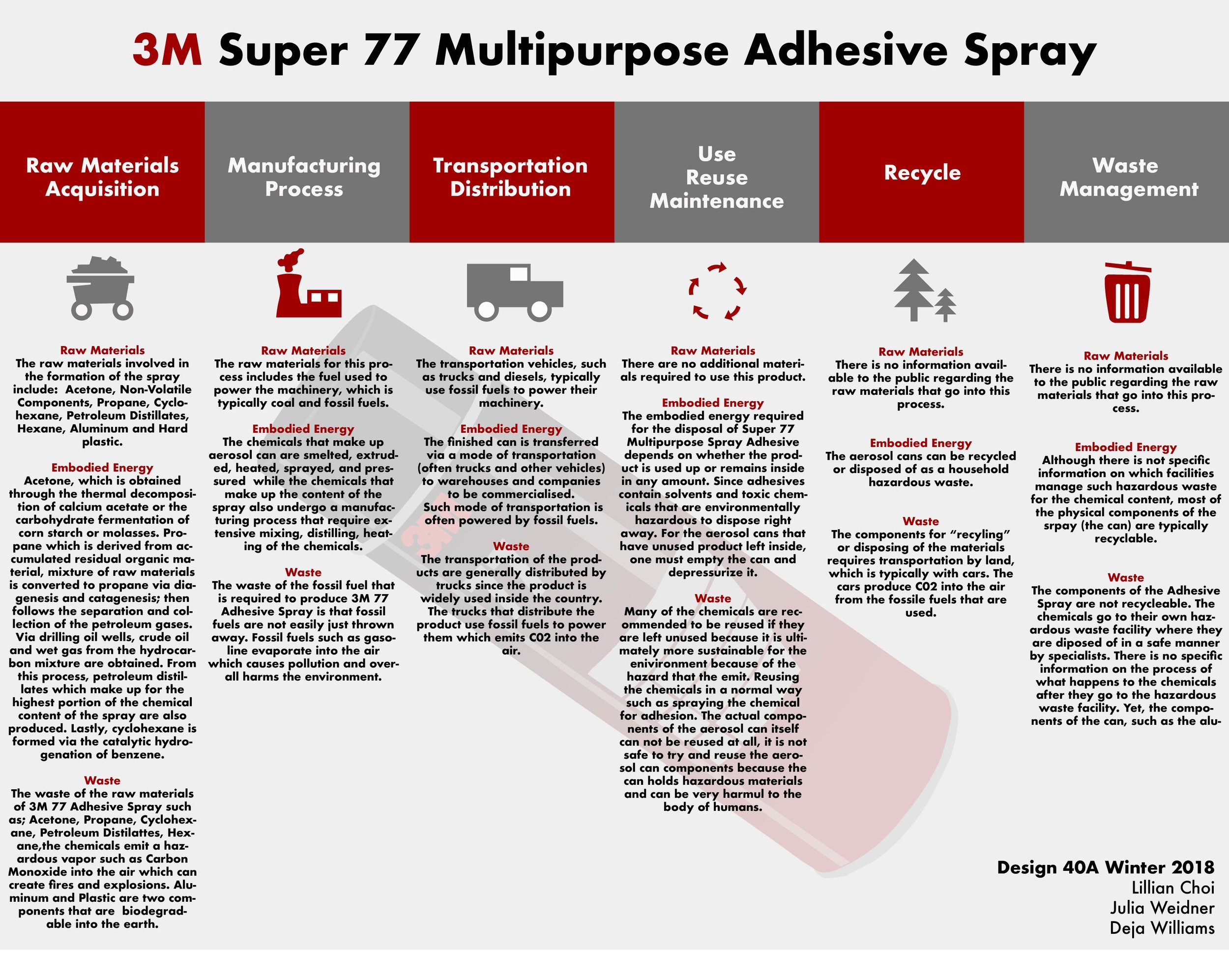
Design Life-Cycle
assess.design.(don't)consume
Julia Weidner
Professor Christina Cogdell
DES 40A
March 15, 2018
Raw Materials for 3M Super 77 Multipurpose Spray Adhesive
In the wake of mass production, which started in the late eighteenth century as a result of the industrial revolution, consumerism has been on the rise in exponential terms. The high demands that human civilization puts on earth’s resources stems from consumerism, and as a society, it is important to take a moment and think about the impacts each and every one of our material goods has. This impact can be traced within the life cycle of a product; this essay will specifically explore the raw material that goes into producing 3M Super 77 Multipurpose Spray Adhesive. 3M is a company which is avid about making the lives of their customers easier.[19] The product Super 77 multipurpose spray Adhesive is not an exception, the product is well rated and practical. Although, it is apparent that 3M’s focus is on creating consumer driven products, and there is a severe environmental impact in the extraction and processing of raw materials for the final product. This product, in particular, is almost entirely composed of derivatives from crude oil. The ingredients of the adhesive are as follows: acetone, non-volatile components, propane, cyclohexane, petroleum distillates, and hexane. These chemicals that make up the adhesive are all conveniently contained within an aerosol can. [1]
Let’s start with the first compound on the list, Acetone. Acetone, also known as 2-propanone or dimethyl ketone (DMK), is a colorless, easy pouring liquid, with a very low
boiling point (so it evaporates easily), which disperses easily in water and organic liquids.[18] Acetone is a secondary material which is manufactured by either the isopropyl alcohol route or the cumene route. The isopropyl alcohol route involves the alcohol being dehydrogenation to acetone over a metal or metal oxide. The cumene route, which is most popular, involves the reaction of benzene and propylene in the presence of catalysts.[4] Benzene and propylene can both be manufactured by one of two processes, either steam cracking of naphtha or the catalytic cracking of petroleum feedstocks. [12][3] Cracking of naphtha is the process of carbenium ion chemistry which converts light hydrocarbons.[4] Catalytic reforming involves naphtha being mixed with hydrogen and fed into a reactor containing a catalyst and operating at 425-530°C and 7-35 barr.[3] Two major companies who manufacture and sell Acetone are Shell Chemical and INEOS. [11][20]
It is important to note that the non-volatile substance of this adhesive is a trade secret and the composition is unfortunately unknown. It could be reasonable to rule out the following substances because the component is categorized as non-volatile: alcohol, mercury, gasoline, and perfume.
The next most abundant component of the adhesive solution is propane. Propane is a naturally occuring gas composed of three carbon atoms and eight hydrogen atoms. Propane is a primary material which is found in petroleum. After the petroleum is extracted from the deep oil fields within the earth, propane is separated from the petrochemicals and refined for commercial use. [15] Some companies who manufacture and sell propane include: Amerigas, Ferrellgas, and Blue Rhino. [6]
Continuing down the list, we have Cyclohexane. Cyclohexane is a clear, colorless liquid with a petroleum-like odor. It can be used to make nylon, as a solvent or used to make other chemicals. [16] Cyclohexane is a secondary material which comes from the catalytic hydrogenation of benzene, which was previously discussed during the breakdown of acetone. [15] Companies that produce cyclohexane include: CEPSA Quimica (Spain) , RCU Chemical, LLC. (US), and Del Amo Chemical Company (US). [5]
Following cyclohexane are petroleum distillates. Petroleum distillates are hydrocarbon solvents produced from crude oil. Examples of these solvents include, mineral spirits, kerosene, white spirits, naphtha, and Stoddard solvent. In addition to being a solvent petroleum distillates “are good for removal of heavy oil and grease, tar, and waxes.” Petroleum distillates are considered a primary material which is refined similarly to propane. [13] Companies that sell petroleum distillates include: Saudi Aramco, CNPC, Sinopec, Kuwait petroleum Corp, Royal Dutch Shell, Exxon Mobil, BP, Total, Bayer, and Ineos. [21]
The last chemical in our long line up is hexane. Hexane is a hydrocarbon molecule found in citrus. Those citrus include: apples, orange juice, guava fruit, roasted filberts, porcini (Boletus edulis), shiitake (Lentinus edodes), heated sweet potato and sage. Hexane is a primary material that is used to extract edible oils from seeds and vegetables, as a special use-solvent, and as a cleaning agent. [9] Some companies that sell and distribute this compound include: Oakwood products, CheMall Corp. MolCore, sigma-aldrich, abcr GmbH, Fisher Chemical, MP Biomedicals, and Parchem. [9]
Now to discuss the prime materials that go into the container which holds all of these, mostly crude oil derived, chemicals. 3M does not explicitly state any product specifications regarding the aerosol can, although it is fair to assume that the can is either made of steel or aluminum. [17] It is a challenge to rule out whether the company would use one material over the other because in terms of cost efficiency, the two materials’ prices continually fluctuate based on the global supply and demand, fuel costs, the availability of iron and bauxite ore. Although, the selection can be narrowed down considering that the material used is most likely chosen due to its corrosion resistance and weight. In this case, aluminum would be the clear stand out because it is less dense than steel, and aluminum doesn’t rust.[10]
Aluminum is the most abundant metal in the earth’s crust, although it is never found free in nature.[7] Aluminum is a primary material, which is extracted from an ore called bauxite. The bauxite is processed and purified to yield a white powder, aluminum oxide, which is dissolved in molten cryolite, then electrolyzed to produce aluminum globules. [8][Lecture 13] The biggest aluminum producers in 2014 included, London Metal exchange, Aluminum Corp. of China, Rio Tinto, China Hongqiao group Lte. and Alcoa. [2]
The last element of final product to consider is the lid and the spray nozzle of the can. Again with reference from standard aerosol cans, since 3M does not explicitly state what the lid and spray nozzle are made out of, it is safe to assume these components are made of hard plastics. Hard plastics are a secondary material, made by chemical polymerization mechanisms after seperating the hydrocarbon chemicals from natural gas, petroleum or coal into pure steams of chemicals. [14]
The raw materials acquisition portion of the lifecycle has been addressed, but it’s important to keep in mind the whole life cycle, which also includes manufacturing, distribution, use/maintenance, recycling, and waste management.
Raw materials do not necessarily play a role in each step of the the life cycle, although they do play a role in places other than just the step of acquisition. Raw materials are needed during the distribution and transportation process in the form of fuel. The fuel is the source of energy for the machines which process and formulate the chemicals that go into the adhesive spray, the machinery used for transportation and distribution of the product’s constituent components and the final product. The standard sources of power in the twenty first century are primarily energy dense sources, such as oil and coal. Oil is extracted from oil fields deep within earth by fracking, whereas coal must be mined.
In order to address all fields of the life cycle, it must be stated that this is a product has a limited number of uses, due to only so much adhesive being in the can, and there is no maintenance. Therefore, there are no materials involved in the maintenance of the product. The research involved regarding the recycling and management of this product indicates that no raw materials that are involved. The treatment of hazardous wastes after they reach a hazardous waste center is extremely vague and does not provide any details regarding whether the chemicals are neutralized with some other chemical.
Now that sufficient energy has been expended on understanding the raw materials of the 3M Super 77 Adhesive spray, its safe to say the plethora of raw materials which must come together to create this final product, are primarily derived from oil and that there are several energy intensive processes which must occur before the final product can come together.
Bibliography
3M. Material Safety Data Sheet. 25 Feb. 2004, multimedia.3m.com/mws/mediawebserver?mwsId=SSSSSuUn_zu8l00x4Y_S58m14v70k17zHvu9lxtD7SSSSSS--
Bell, Terence. “The 10 Biggest Aluminum Producers 2014.” The Balance, The Balance, 21 Nov. 2016, www.thebalance.com/the-10-biggest-aluminum-producers-2014-2339724.
“Benzene Production and Manufacturing Process.” Trusted Market Intelligence for the Global Chemical, Energy and Fertilizer Industries, ICIS, 1 Nov. 2017, 13.11, www.icis.com/resources/news/2007/11/01/9075160/benzene-production-and-manufacturing-process/.
Corma, A., et al. “Light Cracked Naphtha Processing: Controlling Chemistry for Maximum Propylene Production.” Catalysis Today, Elsevier, 26 Aug. 2005, www.sciencedirect.com/science/article/pii/S0920586105003494. (5.5)
“Cyclohexane Suppliers .” Cyclohexane Manufacturers, Cyclohexane Suppliers,Cyclohexane Wholesalers, World of Chemicals , www.worldofchemicals.com/chemicals/chemical-suppliers/cyclohexane.html.
“Cyclohexane (CX) Production and Manufacturing Process.” Trusted Market Intelligence for the Global Chemical, Energy and Fertilizer Industries, 1 Nov. 2017, www.icis.com/resources/news/2007/11/01/9075208/cyclohexane-cx-production-and-manufacturing-process/.
Gagnon, Steve. “It's Elemental.” It's Elemental - The Element Aluminum, Jefferson Lab, education.jlab.org/itselemental/ele013.html.
“GCSE Bitesize: Extraction of Aluminium.” BBC, BBC, www.bbc.co.uk/schools/gcsebitesize/science/add_ocr_pre_2011/chemicals/extractionmetalsrev3.shtml.
“Hexane.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, 7 Mar. 2018, pubchem.ncbi.nlm.nih.gov/compound/hexane#section=Top.
Hornbacher, Adam. “Steel versus Aluminum Weight, Strength, Cost, Malleability Comparison.” Wenzel Metal Spinning, www.wenzelmetalspinning.com/steel-vs-aluminum.html.
ICIS. “Acetone Production and Manufacturing Process.” Trusted Market Intelligence for the Global Chemical, Energy and Fertilizer Industries, 1 Nov. 2007, www.icis.com/resources/news/2007/11/01/9074860/acetone-production-and-manufacturing-process/.
Lazonby, John. “Propene (Propylene).” The Essential Chemical Industry Online, 26 Jan. 2017, www.essentialchemicalindustry.org/chemicals/propene.html.
“Petroleum Distillates Orverview.” Petroleum Distillates - Overview, SAGE Material, 1995, infohouse.p2ric.org/ref/19/18161/alt.cfm-id=pd&cat=ov.htm.
“Plastics.” How Plastics Are Made, plastics.americanchemistry.com/How-Plastics-Are-Made/.
“Propane.” How Products Are Made, www.madehow.com/Volume-3/Propane.html.
PubChem. “HEXANE.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/hexane#section=Top
RecycleNation. “How to Recycle Aerosol Cans.” RecycleNation, 29 Apr. 2014, recyclenation.com/2014/04/recycle-aerosol-cans/.
Shell. Shell Chemicals: Technical Datasheet . Mar. 2016, www.shell.com/business-customers/chemicals/our-products/acetone/_jcr_content/par/textimage.stream/1459949210617/9845e70cbac6be4eafdf87530fb75669b15cc2016e80e20902f0fa11fc2d43a7/acetone-u8903.pdf+.
Shell Global. “Acetone.” Shell Global, www.shell.com/business-customers/chemicals/our-products/acetone.html.
“The World's Largest Producer of Phenol and Acetone.” INEOS Phenol- World's Largest Producer & Supplier of Phenol & Acetone., www.ineos.com/businesses/ineos-phenol/.
“Top Petrochemicals Companies.” Top Petrochemicals Companies - Energy Business Review, EBR, 4 Aug. 2017, refiningandpetrochemicals.energy-business-review.com/news/top-petrochemicals-companies-5892396.
Lillian Choi
Professor Christina Cogdell
DES 40A
3M Super 77 Multipurpose Adhesive Spray: Embodied Energy
3M, a multinational conglomerate was founded in 1902. Since then, the company has contributed to many opportunities in the fields of community, education, and other volunteer works while creating and selling products that make consumers’ lives easier at the same time (3M). One of the company’s best-selling products, Super 77 Multipurpose Spray Adhesive is suitable for bonding materials such as foam, fiberglass, paper, wood, and more [2]. It is composed mostly of petroleum distillates and undergoes intensive production processes. Not only does the content of the spray require a tremendous amount of energy during its production, but the production of the aerosol spray can, transporting of the finished products, and other steps also embody lots of energy.
There are multiple components that go into producing Super 77 Multipurpose Spray Adhesive which require extensive energy: the aerosol can, the chemicals, and the waste after use.
The aerosol can of Super 77 Multipurpose Spray Adhesive, for example, is composed of raw materials in which the main component of an aerosol can is aluminum which is extracted from aluminum bauxite [6]. From 4lbs of bauxite, approximately 1lb of aluminum is obtained, then dissolved using caustic soda. In this smelting process, other chemical elements such as carbon, sodium, and fluorine are involved. The smelting process requires extensive energy in which the production of 1lb of aluminum equates 15kWH of electricity [13]. During the extrusion process from an aluminum sheet, discs are punched out and with the impact from a high-speed reciprocating punch, a closed end can is formed. After the extrusion process, excessive irregular edges are trimmed to make the can into the even height. Then, the inside and outside of the aluminum can are sprayed with laquer and the entire can is cured with heat, then dried. Throughout the swaging process, the top edge of the can is pressured in approximately 15 steps to form a smooth top and roll flange to accept the aerosol valve or spray mechanism. At the final stage, the aluminum can undergoes a pressure tester, which automatically rejects any defective cans. Lastly, the finished can is transferred via a mode of transportation (often trucks and other vehicles) to warehouses and companies to be commercialized [9].
The chemical content of Super 77 Multipurpose Spray Adhesive is comprised of raw materials with high percentages of acetone, propane, cyclohexane, and petroleum distillates [1]. Acetone, which is obtained through the thermal decomposition of calcium acetate or the carbohydrate fermentation of corn starch or molasses [3]. Propane which is derived from accumulated residual organic material, mixture of raw materials is converted to propane via diagenesis and catagenesis; then follows the separation and collection of the petroleum gases [14]. By drilling oil wells, manufacturers obtain crude oil and wet gas from the hydrocarbon mixture in the underground oil fields. From the overall mixture of oil and gas that is procured from the extraction, about 5% of it is propane while the rest are butane, methane and isobutane [11]. From this process, petroleum distillates which make up for the highest portion of the chemical content of Super 77 Multipurpose Spray Adhesive are also produced [12]. Lastly, cyclohexane is formed via the catalytic hydrogenation of benzene. In order to produce the actual content of the spray, however, a manufacturing process in which the chemicals undergo extensive mixing, distilling, and heating to become the final product, is required [15].
The embodied energy required for the disposal of Super 77 Multipurpose Spray Adhesive depends on whether the product is used up or remains inside in any amount [8]. Since adhesives contain solvents and toxic chemicals that are environmentally hazardous to dispose right away. For the aerosol cans that have unused product left inside, one must empty the can and depressurize it. Then, the aerosol cans can be recycled or disposed of as a household hazardous waste [10]. The energy exerted in emptying, depressurizing, recycling, and transporting the finished product is significant.
Sources
“3M Material Safety Sheet.” 3M Company.
“About 3M.” About 3M | 3M United States, 3M United States, www.3m.com/3M/en_US/company-us/about-3m/.
“Acetone Production and Manufacturing Process.” Trusted market intelligence for the global chemical, energy and fertilizer industries, ICIS, www.icis.com/resources/news/2007/11/01/9074860/acetone-production-and-manufacturing-process/.
“Acetone.” Shell Global, Shell Global, www.shell.com/business-customers/chemicals/our-products/acetone.html.
“Aerosol spray.” Wikipedia, Wikimedia Foundation, 9 Dec. 2017, en.wikipedia.org/wiki/Aerosol_spray#cite_note-7.
“Bauxite.” Bauxite | The Aluminum Association, The Aluminum Association, www.aluminum.org/industries/production/bauxite.
“Cyclohexane (CX) Production and Manufacturing Process.” Trusted market intelligence for the global chemical, energy and fertilizer industries, ICIS, www.icis.com/resources/news/2007/11/01/9075208/cyclohexane-cx-production-and-manufacturing-process/.
“Dispose Of Adhesives, Aerosols, Household Cleaners And Other Hazardous Waste Safely.” 57 Ways - 26. Dispose Of Adhesives, Aerosols, Household Cleaners And Other Hazardous Waste Safely, University of Illinois Extension, www.thisland.illinois.edu/57ways/57ways_26.html.
“How Aluminium Aerosol Cans Are Made.” Metal Packaging Manufacturers Association.
“How to Recycle Aerosol Cans.” Earth911.Com, Earth911, earth911.com/recycling-guide/how-to-recycle-aerosol-cans/.
Lichtarowicz, Marek. “Extracting crude oil and natural gas.” The Essential Chemical Industry online, University of York Centre for Industry Education Collaboration, www.essentialchemicalindustry.org/processes/extracting-oil-and-natural-gas-fracking.html.
“Petroleum Distillates.” Petroleum Distillates - Overview, Solvent Alternative Guide , infohouse.p2ric.org/ref/19/18161/alt.cfm-id=pd&cat=ov.htm.
MadeHow. “Aluminum.” How Products Are Made,
http://www.madehow.com/Volume-5/Aluminum.html.
MadeHow. “Propane.” How Products Are Made, MadeHow. www.madehow.com/Volume-3/Propane.html.
MadeHow. “Super Glue.” How Products Are Made,
http://www.madehow.com/Volume-1/Super-Glue.html.
Deja Williams
Professor Christina Cogdell
Des 40A: Section A02
February 8, 2018
Waste: 3M 77 Adhesive Spray
When it comes to sustainability of the environment, many opt to use products that serve a purpose to the well being of the Earth. Yet, people do not realize how the simplest products that they utilize everyday, can be the most harmful to the environment when it comes to the waste of the product. 3M is a product company that is animate about making the lives of the consumers that use their products easier and more efficient. Products such as, 3M 77 Adhesive Spray, are utilized by many designers and people on a day to day bases to put up papers, posters, and fliers. The adhesive spray is perceived as a harmless and very useful product, which spares time and works tremendously better than tape or glue when putting together projects, yet the truth behind the adhesive spray is that it consists of many hazardous materials. 3M 77 Adhesive Spray consists of acetone, propane, cyclohexane, petroleum distillates, hexane and incased in a aerosol can. [20] When it comes to the disposal and waste of these ingredients, they each have their own special procedure that has to be done in order to ensure that they are not harmful to the environment.
The ingredients and materials used for 3M 77 Adhesive Spray have all been deemed as “hazardous”, each of the chemicals are flammable, leak dangerous concentrations, and can spontaneously combust. [3] These ingredients can not just go into a regular trash bin, they have to be disposed into a hazardous waste disposal facility, with leak proof, sealed containers with the name of the chemical properly written on the label of the container. [3] Chemicals are then sorted by their waste type; flammable, acidic, alkalis, or poisons. They are then inspected daily for leaks or any containers that are open. People that have hazardous chemicals or materials can request a pick up to by the Environmental Program Division that they have in their area. [16] This entire process requires the use of trucks and vehicles which requires the emission of CO2 from the fossil fuels that burns when the trucks are being used to transport the waste of the chemicals.
The first ingredient of the Adhesive Spray is Acetone. Acetone, as many women may know, is a liquid chemical mostly used in the removal of nail polish. It is also used as a glassware cleaner and can be distilled with a purity of eighty-eight percent. [9] “Acetone is a colorless, flammable liquid that evaporates easily. It is deemed as an organic compound because carbon atoms are in acetone’s chemical being. Acetone can also be called a ketone, which is a organic compound in a carbonyl group paired by two hydrocarbon groups.” [7] Acetone may also be recognized as a solvent because of the chemicals ability to dissolve other materials, which secures it’s place in the “Hazardous Chemicals” category. When disposing of acetone it is safest not to dispose of the chemical down a drain as it can be very dangerous to the environment. [4] I could not find exact information on the waste and disposal of acetone, but based on the Hazardous Waste Management Book there are multiple ways of disposing of these hazardous chemicals such as acetone with land disposal, thermal methods, solidification and biological methods. [10]
Propane is widely known as one of the most flammable chemicals that is sold as a regular retail item that many people utilize, most commonly used in heat applications, for example, cooking. “Propane is a flammable hydrocarbon gas liquefied through pressurization. Propane comes from natural gas processing and oil refining. It is used as heating, cooking and auto fuel” [8] Propane is a fossil fuel which is made by oil refining and natural gas processing, it also uses refreeze ration to separate unprocessed natural gases. [8] Most of the chemicals I can not find the exact waste method for them but based on reading of disposal of chemicals, I know the the process consists of labeling properly, emptying the contents in a safe way, best way is to use up all the contents or reusing them. There is not really a “extremely safe” process of disposing hazardous chemicals.
Cyclohexane is a chemical that is not commonly known by anyone other than those that study it. “Cyclohexane is a clear colorless liquid with a petroleum-like odor. Used to make nylon, as a solvent, paint remover, and to make other chemicals. Flash point -4°F. Density 6.5 lb / gal (less than water) and insoluble in water. Vapors heavier than air.” [12] If cyclohexane is spilled or leaking, releases a vapor, carbon dioxide or carbon monoxide, that ignites fires if it comes in contact with strong oxidizer and has to be contained with dry chemical, foam or carbon dioxide, water will only make the fire bigger. When handling cyclohexane you have to wear protective clothing over your skin because the result of skin contact with cyclohexane can be deadly. To dispose of the chemical it is best to either reuse it or recycle it properly and sent to a hazardous wast facility that is approved by RCRA (Resource Conservation and Recovery Act). [1]
Petroleum Distillates sounds like something that is commonly used as a household item, Petroleum Jelly or Vaseline, but that is not what Petroleum Distillates are. Petroleum Distillates is a liquid chemical that has a kerosene or gasoline like odor. [13] Petroleum Distillates are extremely dangerous if they are inhaled or come into contact with skin. The chemical can affect the nervous system as well as the liver and kidneys. Petroleum Distillates are also an extremely flammable chemical due to the vapor that is produce from the chemical and requires dry chemicals, CO2 and alcohol resistant foam to put out. The gas produced from a petroleum distillate fire is extremely poisonous. They react violently with Nitrogen, Chlorine, Bromine, Nitric Acid, and Peroxides to name a few. When disposing of Petroleum Distillates it is best to keep them in a tight container that is well ventilated and to avoid flames or heated areas. [17] The container containing the liquid is then disposed of in a hazardous waste facility.
Hexane is considered as a chemical solvent and is widely used as a cleaning agent, as well as a solvent for glues and adhesives. It is mostly used as the liquid in low temperature thermometers. [4] The most common exposure to hexane is by inhaling it which is extremely dangerous for humans because its can cause mild depression, dizziness, nausea and headaches. Hexane is not as flammable as the other chemicals in 3M 77 Adhesive Spray, but it is sill a flammable substance. The flammable limits depend on the volume of pure hexane but it still pleases a flammable vapor which can be contained by carbon dioxide or dry chemicals. Hexane is expected to quickly evaporate in water and on land and the vapor that is released from the evaporation is hazardous. [14] when disposing of hexane what every can not be saved has to be disposed of in a hazardous waste facility.
Aerosol Cans are widely used to hold many substances such as paint, glues, and pesticides and are deemed as hazardous if they still contain any chemicals or hazardous liquids in them, which then leads to the process of hazardous waste disposal. If the Aerosol Can is empty of all hazardous liquids then it can be disposed of regularly. Determining if a Aerosol can is empty is a process of its own. It has to pass certain guidelines to be considered a empty can. Guidelines such as determining if the chemicals in the can are “acute hazardous” or hazardous. Aerosol Cans are made up of Aluminum and Hard Plastics. “aluminum is the most abundant metal in the earth's crust, it is never found free in nature” [5] Which means that the process to get aluminum considered drilling which ultimately harms the earth and alot of machinery which run off of electricity. “Plastics, also called polymers, are produced by the conversion of natural products or by the synthesis from primary chemicals generally coming from oil, natural gas, or coal.” [15] Plastics derive from fossil fuels which are detrimental to the well being of the Earth because they are not easily recyclable or disposed of. Back to the can as a whole, to consider a Aerosol can with hazardous chemicals empty, the can has to have the chemicals disposed of by “normal means” which means, for example, that the can has to be disposed of by spraying the contents out of the can during use of the product. [2] The can cannot have more that 3 percent or one inch of residue left over. [2] Another method of disposal of the aerosol can is by a cooling method. The cooling method consists of cooling the dispenser and contents to a temperature below the boiling points of the propellant which makes the substance turns to vapor pressure. The propellant is removed from the container and then disposed of in containers or reused and the can is then disposed of regularly. [11]
References
1. Baker, J.T. “Material Safety Data Sheet: Cyclohexane” http://terpconnect.umd.edu/~choi/MSDS/J.T.Baker/CYCLOHEXANE.htm
2. Bottone, Rosanne (2016, April 26) “How to Manage Aerosol Cans Under RCRA Hazardous Waste Rules” https://www.lion.com/lion-news/april-2016/how-to-manage-aerosol-cans-under-rcra-hazardous-wa
3. Duke University, Chemistry Administration “Disposal Procedures”. https://chem.duke.edu/about/disposal-procedures
4. Environmental Protection Agency, United States (1999, December) “Hexane” https://www.epa.gov/sites/production/files/2016-09/documents/hexane.pdf
5. Gagnon, Steve. “It's Elemental.” It's Elemental - The Element Aluminum, Jefferson Lab, education.jlab.org/itselemental/ele013.html.
6. Garcia, Nissa “What is Acetone - Structure, Uses, and Formula” [study.com] https://study.com/academy/lesson/what-is-acetone-structure-uses-formula.html
7. Gaskin, R.E. (2005, August) “Characterising Plant Surfaces For Spray Adhesion and Retention” [Academic Journal] https://www.cabdirect.org/cabdirect/FullTextPDF/2005/20053189311.pdf
8. Hahn, Eric “What is Propane Gas?” [Blog Post] http://www.elgas.com.au/blog/1689-what-is-propane-gas
9. Hammond,Karen (2011, October 4) “Recycling of Waste Acetone by Fractional Distillation” [Academic Journal] https://pubs.acs.org/doi/abs/10.1021/ed2001158
10. LaGrega, Michael D (2001) “Hazardous Waste Management: Second Edition” [book] https://books.google.com/books?hl=en&lr=&id=NdYYAAAAQBAJ&oi=fnd&pg=PR3&dq=waste+of+hazardous+chemicals+&ots=EaNZI4kW3q&sig=79ggVOfW9ySst9rYpIs2jOhXYpQ#v=onepage&q=waste%20of%20hazardous%20chemicals&f=false
11. Nash, John P (1989, December 7) “Method for Disposal of Aerosol Can” https://patents.google.com/patent/US5088526A/en
12. National Center for Biotechnology Information. (2004, September 16) “PubChem Compound Database” https://pubchem.ncbi.nlm.nih.gov/compound/8078
13. New Jersey Department Of Health (2011, August) “Hazardous Substance Facts Sheet: Petroleum Distillates” http://nj.gov/health/eoh/rtkweb/documents/fs/2648.pdf
14. Philipine Prosperity Chemicals Inc (2009, September 28) “Material Safety Data Sheet: Hexane” http://www.ppci.com.ph/msds2k10/06_hexane.pdf
15. “Plastics.” How Plastics Are Made, plastics.americanchemistry.com/How-Plastics-Are-Made/.
16. “Procedures for Laboratory Chemical Waste Disposal” https://www.mun.ca/sgs/current/chem_waste.pdf
17. Rutgers University, Environmental Service Group. “Hazardous Waste Disposal Policy/Procedures” http://rehs.rutgers.edu/pdf_files/hazwaste_disposal.pdf
18. SAGE (1995, March 17) “Solvent Alternatives Guide” http://infohouse.p2ric.org/ref/19/18161/alt.cfm-id=pd&cat=ov.htm
19. Vanderbilt University (2018) “Environmental Health and Safety” https://www.vumc.org/safety/waste/chemical-waste-sewer-disposal
20. “3M Safety Data Sheet” https://multimedia.3m.com/mws/mediawebserver?mwsId=SSSSSuUn_zu8l00x482xN8mvNv70k17zHvu9lxtD7SSSSSS--