Design Life-Cycle
assess.design.(don't)consume
Kris Tian
Professor Christina Cogdell
DES 40A
Winter Quarter 2018
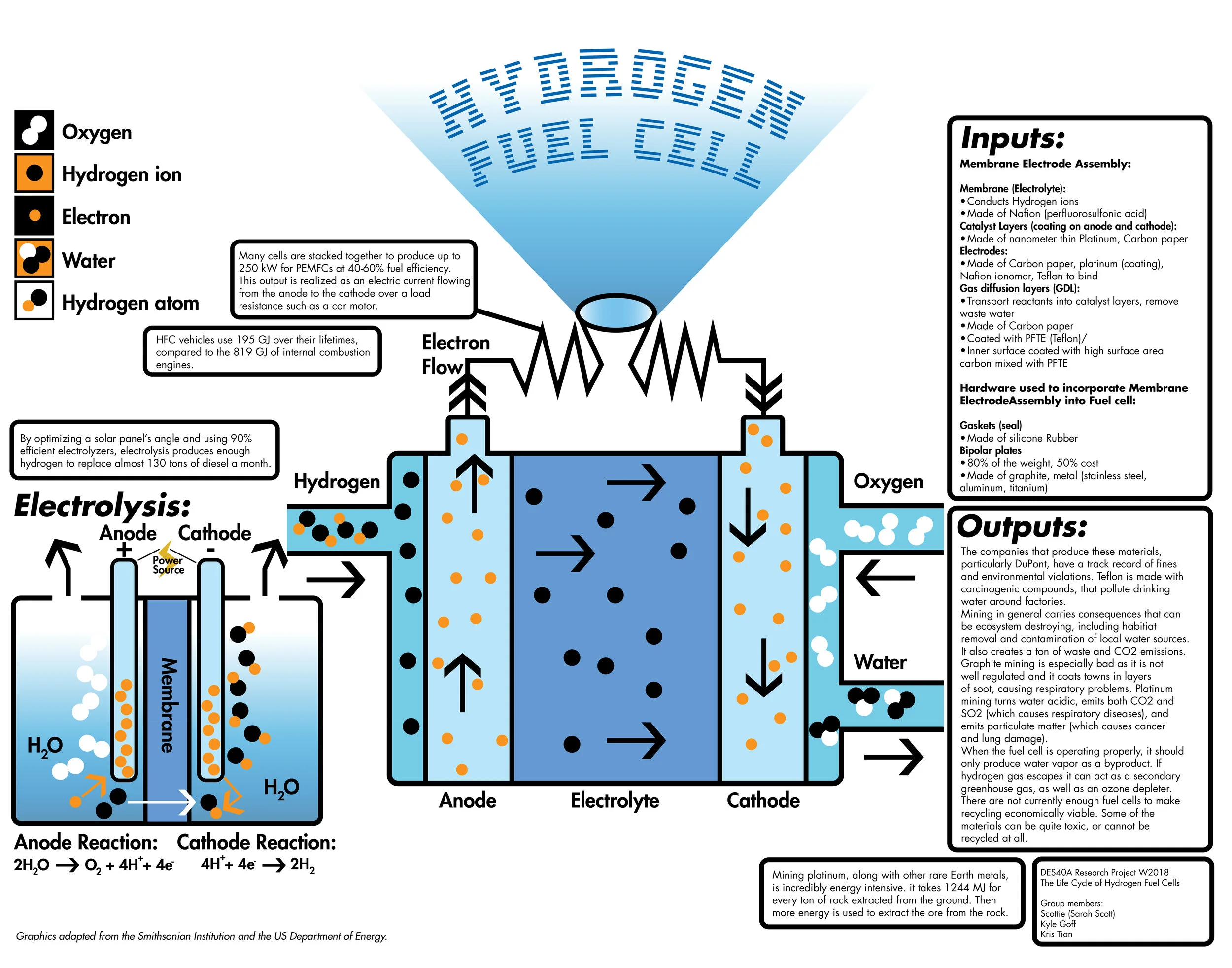
Raw Materials for Proton Exchange Membrane Fuel Cells
The Proton Exchange Membrane Fuel Cells (PEMFCs) are one of the main focuses of the current quest for new energy sources due to its relatively low waste production. Also, because of the strict environments where PEMFCs operate, certain mechanical and chemical criteria must be satisfied by the selected raw materials so that the fuel cells can function normally and efficiently. Therefore, the selection of materials for PEMFCs is very crucial. Although the current PEMFCs produce adequate results, improvements can still be made in areas of efficiency, cost and durability of the fuel cells by making changes to the current choice of raw materials.
As the global awareness of the environmental problems increases drastically in the recent years, the world’s attention is shifting toward the research and development of technologies with clean energy, low to zero emissions, low cost and high efficiency. One of the leading technologies that can satisfy these criteria the best and eventually be integrated into the daily consumption by the majority of the world’s population is Hydrogen fuel cell technology. Thanks to the rapid growth in the advanced technologies in the recent years, various types of hydrogen fuel cells have been found to produce some acceptable results as new energy sources, one of which—the proton exchange membrane fuel cell (PEMFC)—is currently the leading focus in the scientific community for fuel cell vehicle applications. PEMFCs “. . . produce electrical energy from the chemical energy of [hydrogen] and oxygen.” (Kraytsberg, 7303) According to the U.S. Department of Energy, they can be broken down into two main parts: the membrane electrode assembly (MEA), and the hardware components—gaskets and bipolar plates; the gaskets are designed to improve the durability by sealing the edges of MEA to prevent gaseous leakage, while the bipolar plates increase the efficiency by connecting individual fuel cells together into stacks. Since PEMFCs are electrochemical devices, improvements will be hard to achieve without careful examination and analysis of the materials used in the current fuel cell models.
In real world applications, if a fuel cell does not have a durable and safe hardware as support and protection, it will fall apart before long regardless of how efficient or advanced it is. Therefore, even though the hardware does not contribute to the electrochemical reactions of the fuel cell, the materials used in the hardware component of the PEMFC stacks still play a significant role.
The first important hardware component are the gaskets, which are used as seal in each fuel cell, and “. . . to keep the reactant gases within their respective regions.” Due to the demanding environment of PEMFCs, gaskets are subject to both chemical and mechanical challenges, such as acidity, humidity carried by the reactant gases—air and hydrogen, high temperature, degradation caused by coolants, and “. . . mechanical compression between the bipolar plates forming the cell.” (Tan et al. 669) Therefore, the material used for gaskets has to be elastomeric as well as durable in order to seal around the edges of MEA for a lengthy period of time. Silicone rubbers are one of the most commonly used materials for gaskets due to their flexibility at low temperature, decent electrical properties, gas permeability, “. . . low cost and easy fabrication.” (Tan et al. 670) However, one of the recent studies have confirmed that Silicones possess some disadvantages in terms of the reliability and durability of PEMFCs. Tan et al. did a few experiments to test out the durability of Silicones and ethylene-propylene-diene-monomer (EPDM) —a synthetic thermoplastic elastomer—in a simulated PEMFC environment, and concluded that Silicones degraded and completely failed after 6 weeks of usage in the simulated fuel cell environment at a temperature of 80 ºC, while EPDM only showed a little degradation after being in the same environment for 45 weeks. This shows that though Silicones once were the most suitable raw material for gaskets at the time, they are not a feasible material to be used in fuel cells for long-term usage nowadays, which also indicates a trend that more and more current materials in the fuel cells will be replaced.
The second important component of PEMFCs hardware are the bipolar (flow field) plates, “. . . which serve as current collectors and supply reagents to the MEA electrodes. . . also electrically connect multiple MEAs in series.” (Kraytsberg, 7304) In addition to that, “. . . bipolar plates serve to keep oxidant and fuel gases separate from one another.” (Haile, 2003) In other words, the ideal material for bipolar plates must have high electrical conductivity, low resistivity, less porosity and high corrosion resistance. According to Kakati and Mohan, all of these properties of ideal bipolar plates will directly affect the overall efficiency and durability of PEMFCs, which is why the bipolar plates rank second in the PEMFC cost and account for 80% of the total weight. Today, bipolar plates are made of materials such as graphite, polymer/graphite composite, carbon/carbon composite and metal. Although metallic bipolar plates are cost effective in large scale production, they have very low corrosion resistance. Even the stainless steels “becomes contaminated by the corrosion products” on the anode side when it is being used, as stated by Steele in his study. One way to solve the corrosion issue is using polymer coating, but the overall cost and weight of the fuel cell will then increase, making metal an undesirable material. On the other hand, graphite being the most common material for the job has low cost and low density, but it is too brittle and porous, and requires mechanical modification, making this material too expensive. As for carbon/carbon composite, though it can satisfy all the criteria for ideal properties to maximize PEM fuel cells’ efficiency and durability, Kakati et al. showed that its high manufacturing cost prevents it from being the optimal choice of material. So polymer/graphite composites are developed to keep the corrosion resistance, and reduce the costs resulted from manufacturing graphite plates thanks to the inexpensive polymers, polypropylene, for instance, according to Cunningham et al. With the enlightenment of the polymer/graphite composite, Kakati et al. investigated into the manufacturing of carbon-graphite-polymer composite, or advanced composite, for bipolar plates. They developed a process to produce the advanced composite by combining natural graphite with synthetic graphite, carbon fiber and carbon black, which resulted in bipolar plates that are cost effective, superiorly conductive “. . . due to the better percolation of carbon black and better distribution of carbon fibres”, and the most suitable for PEMFCs thus far.
The most important component of the PEM fuel cell is the MEA, which includes catalyst layers, electrodes, gas diffusion layers (GDLs) and the heart of the PEMFC technology—the polymer electrolyte membrane, or PEM, also called a proton exchange membrane. As stated before, while bipolar plates are the second most costly part of a fuel cell, “[a] recent technical cost analysis indicates that the cost of platinum electrode account for about 50% of the PEM fuel cell cost”. (Kakati et al. 45) Platinum is the primary source of raw material used for the catalyst layers of the PEMFCs. When the first generation of PEMFCs came out and were put into application, large amount of platinum was used in the fuel cells, driving the production cost beyond the acceptable amount, which explains why fuel cells were not initially included in the commercialization. Today, a significant portion of research on the optimization of PEMFCs is still put toward reducing the amount of platinum used in catalyst layers, where the half-cell reactions take place. Compared to the first generation of PEMFCs, the loading of platinum catalyst supported by the polytetrafluoroethylene (PTFE) has been reduced to less than one hundredth of the original amount, which significantly lowered the cost of PEMFCs, according to Litser and McLean. Furthermore, since platinum is available in the form of its native metal, platinum-bearing copper-nickel ores, and an alloy with iron, extraction and refining processes are needed in the process of producing platinum with acceptable purity. Generally, the more complex the process of obtaining the material is, the more expensive that material costs. Therefore, to avoid increasing the price of platinum, “a very high proportion of the platinum metals used in industry is refined and reused repeatedly, often without change of ownership. . . A considered estimate of the total amount of platinum in circulation in the world today would be between 25 and 30 million troy ounces.” (Hunt, 126)
Furthermore, two other important parts of the MEA that are in direct contact with the catalyst are electrodes and GDLs, which share the same commonly used material—carbon paper, because its porosity allows effective removal of waste water and transport of reactant gases among the triple boundaries of GDL, CL and the electrode. (Litser, 2004) This in turns plays a role in ensuring the stability and durability of PEMFCs.
Finally, in PEM—the core of the functionality of PEMFCs, a synthetic ion-conducting polymer, or ionomer, called Nafion is used. It is “produced by the E. I. DuPont Company. These materials are generated by copolymerization of a perfluorinated vinyl ether comonomer with tetrafluoroethylene (TFE).” (Mauritz, 4535) Just like bipolar plates, the material used for PEM has to satisfy a range of requirements for the fuel cell to have efficient and stable performance over a long period of time. The one unique requirement for the material in PEM is to function as a “proton-conducting gas barrier” (Kraytsberg, 7306) that only allows the transport of proton to cathode. Thus far, according to Steele, Nafion and its related chemicals are preferred choice of materials used in PEMFCs. The only issue being the high cost to synthesize Nafion, that is why “materials currently being used in PEMFC. . . remain the same as those selected at least 25 years ago.” (Steele, 346)
One thing that is worth noting is that although the PEM fuel cells are the leading example of clean energy sources, it does not mean that they do not possess any downside at all. Sometimes, in order to achieve economic efficiency, some sacrifice has to be made. For example, “[a]ir is the most practical and economical way to feed the fuel cell stack. However, air pollutants. . . contaminate the fuel cell, resulting in MEA damage and performance degradation.” (Cheng et al., 741) Also, there is no particular information mentioned about the materials used for transportation of fuel cells, perhaps it is because they are not as important in comparison to the other aspects of the life cycle.
In conclusion, after the examination of materials used in some of the most important parts of PEM fuel cells, it is clear that fuel cell technology is still in the phase of rapid growth, and that though there are several optimizations that can be made in the material selection, it will take time to find the right material without destroying the intricate balance between the economics, performance and environmental effects of fuel cell technology. With that being said, the future of PEM fuel cells is quite optimistic.
Bibliography
Cheng, Xuan, et al. "A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation." Journal of Power Sources 165.2 (2007): 739-756.
Cunningham, B. D., et al. “Review of materials and processing methods used in the production of bipolar plates for fuel cells.” International Materials Reviews, vol. 52, no. 1, 2007, pp. 1–13., doi:10.1179/174328006x102556.
Haile, S. (2003). Fuel cell materials and components☆☆☆The Golden Jubilee Issue—Selected topics in Materials Science and Engineering: Past, Present and Future, edited by S. Suresh. Acta Materialia, 51(19), pp.5981-6000.
Hunt, L. B., and F. M. Lever. “Availability of the Platinum Metals.” Johnson Matthey Technology Review, 25 Feb. 2016, www.technology.matthey.com/article/13/4/126-138/.
Kakati, B. K., and V. Mohan. “Development of Low‐Cost Advanced Composite Bipolar Plate for Proton Exchange Membrane Fuel Cell.” Fuel Cells, WILEY‐VCH Verlag, 14 Feb. 2008, onlinelibrary.wiley.com/doi/10.1002/fuce.200700008/full.
Kraytsberg, Alexander, and Yair Ein-Eli. “Review of Advanced Materials for Proton Exchange Membrane Fuel Cells.” ACS Publications, 21 Oct. 2014, pubs.acs.org/doi/abs/10.1021/ef501977k.
Litster, S, and G McLean. “PEM Fuel Cell Electrodes.” Journal of Power Sources, Elsevier, 2 Mar. 2004, www.sciencedirect.com/science/article/pii/S0378775304000631.
Mauritz, Kenneth A, and Robert B Moore. “State of Understanding of Nafion.” ACS Publications, American Chemistry Society, 21 Sept. 2004, pubs.acs.org/doi/full/10.1021/cr0207123#citing.
“Parts of a Fuel Cell.” U.S. Department of Energy, www.energy.gov/eere/fuelcells/parts-fuel-cell.
Steele, Brian CH, and Angelika Heinzel. "Materials for fuel-cell technologies." Materials For
Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group. 2011. 224-231.
Tan, Jinzhu, et al. “Degradation of elastomeric gasket materials in PEM fuel cells”. Materials Science and Engineering: A, Elsevier. 3 Nov. 2006, https://www.sciencedirect.com/science/article/pii/S0921509306020867#aep-section-id27
Kyle Goff
Professor Christina Cogdell
DES 040A
Winter Quarter 2018
Energy in Hydrogen Fuel Cells: A Vehicle-Focused Life Cycle Analysis
In 2017, car manufacturer Toyota released the Mirai. This vehicle, unlike its rivals with combustion engines or electric batteries, runs solely on hydrogen. Hydrogen fuel cells (HFCs) are not a new idea, but recent interest and advances in automotive technology have brought them back to the forefront of power solutions promising a more sustainable future. Since they take hydrogen as an input and output water as a byproduct, they are seen as a clean energy solution.
As a new technology, HFCs have many years of development left to reach their full potential, but they already seem promising. Although hydrogen fuel cells use a significant amount of energy at each stage of the lifecycle, they effectively use resources because they have high efficiencies while still providing sufficient power output for practical applications.
There are a few types of HFCs, but the most common and practical are proton exchange membrane fuel cells (PEMFCs). The basic components of PEMFCs are electrodes and bipolar plates made from conductive materials, a gas diffusion layer, a catalyst made from platinum or noble metals, and an electrolyte membrane (Litster 62). Most fuel cell types work by combining pure hydrogen with pure oxygen from the air to convert their chemical energy to electricity with water as a byproduct. Each cell uses little material, but a single cell is not enough to make a useful amount of power for modern applications. Many cells are stacked together to produce up to 250 kW for PEMFCs at 40-60% fuel efficiency (EEREIC). This output is realized as an electric current flowing from the anode to the cathode over a load resistance such as a car motor. This seems like an ideal product for low emissions; however, the energy required to obtain these materials contributes significantly to the energy use over a fuel cell’s life cycle.
One primary material in PEMFCs is platinum, which requires energy to mine, purify, and transport. Platinum is a catalyst in the chemical reaction. A catalyst is a material that is used to speed up or reduce activation energy required for a reaction to take place, increasing efficiency (EEREIC). Mining platinum, along with other rare Earth metals, is incredibly energy intensive. At the Northam Platinum mine in South Africa as of 2009, it takes 1244 MJ for every ton of rock extracted from the ground. Then more energy is used to extract the ore from the rock. To make matters worse, the primary energy used is electricity, a secondary energy source (Glaister 444). Fortunately, research has been done to minimize the use of platinum in cells. Some of the first PEMFCs used up to 4 mg/cm2, but new methods have reduced this to 0.014 mg/cm2 through a process called sputtering (Litster 62). New breakthroughs are attempting to eliminate the reliance on platinum and other rare metals (Chen) which should eventually reduce the energy input from platinum as PEMFCs become more commercially available.
Hydrogen fuel cells are comprised of many synthetic materials. Composites of carbon, like graphite, are usually used for the bipolar plates. (Steele 346). Another carbon product used is carbon paper. While sharing the name of the paper used to make carbon copies, it is a porous material specific to the fuel cells made. Its carbon microfibers facilitate the reaction by providing a gas diffusion layer (Litster 62). Little to no information exists on the manufacturing energies of these synthetic materials. Due to the large number of manufacturers with many similar materials used with unknown percentages in the market, finding concrete numbers on energy input for any one material is difficult. One last common synthetic material is Nafion, a registered trademark material produced by the Chemours division of DuPont at various locations around the United States. It is used as the proton exchange membrane in the HFC (Steele 348). Like before, the energy required to produce Nafion is not disclosed. So, for many of the synthetic materials used in hydrogen fuel cells, information regarding energy consumption is unavailable and should be further researched since most studies so far focus on HFC cars as a whole.
The last important component of hydrogen fuel cells is, of course, hydrogen. Hydrogen is not readily available in its pure form, though, so it can be obtained through the chemical process of electrolysis. Electrolysis uses electrical energy to split water molecules into hydrogen and oxygen. The hydrogen is then stored in tanks until pumped into a fuel cell. Electrolysis can be highly efficient, making excellent use of its energy inputs. This, however, is secondary electrical energy, often obtained from burning primary energies like coal. Fortunately, it may be possible to remove the reliance on fossil fuels for electrolysis according to a study done in Iran on a photovoltaic electrolysis station. By optimizing the solar panels angle and using 90% efficient electrolyzers, they produced enough hydrogen to replace almost 130 tons of diesel a month (Fereidooni 422). These numbers are possible by using PEM electrolyzers which are simple devices capable of generating 99.999% pure hydrogen from only solar energy (Barbir 668).
The energy in transporting each material used in a PEMFC varies greatly but contributes substantially to its life cycle. As of now, “natural gas is transported through pipeline or road tankers to decentralized refuelling stations, where it is produced through steam reforming,” and results in 770 MJ consumed for every GJ worth of hydrogen produced (Hussain 2296). Looking at HFC vehicles as a whole, the energy required to produce, assemble, and distribute the vehicle is about 81 GJ and is comparable to internal combustion engine vehicles (2298). Since this considers automotive materials as well, the number can be seen as an upper limit on the energy required for manufacturing the fuel cell up until it is sold. An example of this transportation energy could be from Nafion, which is produced in the United States but processed at manufacturing plants worldwide. Additionally, almost all metal mining occurs overseas, especially in South Africa as mentioned before. A large amount of energy is used in physically transporting materials to manufacturing sites in various countries around the globe.
During normal operation, a hydrogen fuel cell makes very efficient use of energy. Most gasoline powered engines lose most of their energy to heat, yielding only 20-40% efficiency depending on engine technology (Ingram). HFCs range in efficiency based on technology, but PEMFCs can reach 40-60%, as previously mentioned. To put energies in perspective, consider again HFC vehicles use 195 GJ over their lifetimes as compared to the 819 GJ of internal combustion engines (Hussain 2298). Even including the production and transportation energy shows that the total energy use over the life cycle of an PEMFC vehicle is 276.3 GJ versus 896.7 GJ for a gasoline powered car (2298). As long as the production of hydrogen fuel can be produced by solar in the future, the embodied energy of a PEMFC vehicle shows promise in reducing global energy usage. The last important step of the life cycle, however, is recycling.
Due to the infancy of hydrogen fuel cell technology, efficient recycling methods have not yet been established. This has led to a global platinum reserve below estimated global reserves (Wittock 356). The key idea as of now is to recover as much platinum as possible to help ensure we do not deplete the resource entirely if the market expands. The collection of fuel cell vehicles for recycling is the most difficult aspect because many fuel cell vehicles end up “in countries lacking the infrastructure required for environmentally sound recycling,” (347). Thus, to recycle effectively as of now would require increasing transportation energy at the end of the life cycle to move the vehicles to countries with recycling plants. Almost no information exists to indicate the energy required in this process versus the energy gained by not mining more platinum. Since platinum is mined in other continents, however, it would again cut down on transportation energies to have local recycling. HFC vehicles in general require the same energy to recycle and dispose of as internal combustion vehicles (Hussain 2296), but how much of this energy is specifically for the fuel cell is unclear.
Although currently there are issues with the energy required to produce HFCs, solutions are on the way. Platinum is being used less frequently in the cells, and hydrogen can be made safely and locally with relatively high efficiency from renewable power. The energy required for a combustion car may be less on the front-end, but the low emissions of a fuel cell vehicle over its lifetime require less energy overall. Given the time and infrastructure to expand the market and recycling process, hydrogen fuel cells could become an excellent alternative to traditional engines, especially in automobiles.
Bibliography
Barbir, Frano. "PEM electrolysis for production of hydrogen from renewable energy sources." Solar energy 78.5 (2005): 661-669.
Chen, Zhongwei, et al. "A review on non-precious metal electrocatalysts for PEM fuel cells." Energy & Environmental Science 4.9 (2011): 3167-3192.
Energy Efficiency and Renewable Energy Information Center. Hydrogen Fuel Cells. Department of Energy, 2006. www.hydrogen.energy.gov/pdfs/doe_fuelcell_factsheet.pdf
Fereidooni, Mojtaba, et al. "A comprehensive evaluation of hydrogen production from photovoltaic power station." Renewable and Sustainable Energy Reviews 82 (2018): 415-423.
Glaister, Bonnie J., and Gavin M. Mudd. "The environmental costs of platinum–PGM mining and sustainability: Is the glass half-full or half-empty?." Minerals Engineering 23.5 (2010): 438-450.
Hussain, M. M., I. Dincer, and X. Li. "A preliminary life cycle assessment of PEM fuel cell powered automobiles." Applied Thermal Engineering 27.13 (2007): 2294-2299.
Ingram, Antony. “Toyota Gasoline Engine Achieves Thermal Efficiency Of 38 Percent.” Green Car Reports, 14 Apr. 2014
Litster, S., and G. McLean. "PEM fuel cell electrodes." Journal of Power Sources 130.1-2 (2004): 61-76.
Steele, Brian CH, and Angelika Heinzel. "Materials for fuel-cell technologies." Materials For Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from
Nature Publishing Group. 2011. 224-231. Wittstock, Rikka, Alexandra Pehlken, and Michael Wark. "Challenges in Automotive Fuel Cell Recycling." Recycling 1.3 (2016): 343-364.
Scottie (Sarah Scott)
Professor Christina Cogdell
DES40a, W2018
The Waste Products of Fuel Cells
As of February 2018, carbon dioxide measurements in the atmosphere reached 407.61 parts per million (NASA). Most scientists agree that the upper safe limit on carbon dioxide is 350 ppm (“Understanding 350”). The consequences of surpassing this limit are in most ways unrealized and unimaginable. We do not yet understand how dramatic the effects of rapid worldwide climate change will be, but we have some idea. More extreme weather, mass extinctions, hundreds of millions of climate refugees, just to name a few costly problems. We don’t know just how dire the consequences of our actions will be, but we do know that we need to do something about it. A keystone of this solution is developing and implementing alternative forms of energy. Hydrogen Fuel Cells are one of the most promising forms of alternative energy, especially with the release of Toyota’s Mirai, the first fuel cell vehicle on the market. But fuel cells are not perfect. Through the fuel cell’s life cycle assessment, or an assessment of all the product’s environmental impacts from its initial materials to its production, to its disposal, it is shown that some aspects of the fuel cell are not sustainable. Despite the many imperfections of the Hydrogen Fuel Cell, the technology is ultimately more environmentally sound than traditional energy sources due to the Fuel Cell’s primary waste product being only water.
Hydrogen fuel cells are based on a simple chemical reaction. Hydrogen flows into the fuel cell and is stripped of its single electron. That electron is then used for power. The hydrogen ion, meanwhile, flows through the membrane and attaches itself to incoming airborne oxygen (O2). They hydrogen and oxygen combine to create water (H2O) and are then released out of the fuel cell. Fuel cells by themselves are completely clean because they produce only water as a byproduct. When the hydrogen fuel is made with renewable energy, such as solar power, the entire process can be completely clean.
The main materials used in fuel cells are Nafion, Teflon, Silicone Rubber, Platinum, Graphite, carbon paper, and carbon fiber. The most popular material used for the PEM membrane is Nafion, originally developed by DuPont, now owned by its spin off company, The Chemours Company. Both DuPont and Chemours have a horrific track record of polluting air and nearby water systems. Their most well-known recent offense is the GenX pollution. Originally given off as hexafluoropropylene oxide dimer acid (HFPO-DA), an air pollutant, this compound contaminates rain and finds its way into nearby streams, and eventually groundwater, contaminating drinking water (Hogue, 2018). GenX does not degrade in nature, and it causes several health problems such as, “increases in cholesterol levels, thyroid and liver disease, pregnancy related high blood pressure (known as pre-eclampsia) and other ailments” (Clabby, 2017). Nafion isn’t particularly unsafe to workers if they follow safety precautions, however, “exposure to thermal decomposition products of Nafion™ perfluorinated membranes may cause a temporary flu-like condition” (Safety, 2016). In addition, Nafion disposal has only one step: into the landfill. “Nafion™ materials are not biodegradable, contain no extractable material, and are unaffected by exposure to sunlight, seawater, or fresh water” (Safety, 2016).
DuPont is also responsible for the production of Teflon. Teflon itself is not hazardous, but Perfluorooctanoic Acid (PFOA), used in the production of Teflon, is carcinogenic. It’s pervasive in both the environment and in humans, so much so “that it is present worldwide at very low levels in just about everyone’s blood” (Teflon, 2016). In Teflon products, there are only trace amounts of PFOA, not enough to be carcinogenic. Exposure levels are only high enough to cause cancer when people either work with the compound in high enough doses or if they live close to a DuPont chemical plant that uses PFOA. There is a massive phase out of PFOA underway, however, the new chemical replacements are “raising concerns” due to the fact that they are “trade secrets” and cannot be tested (Kelly, 2016).
Silicone Rubber is manufactured almost exclusively by an oligopoly containing Dow Chemical, Wacker Chemie, and Shin-Etsu Chemical. Dow Chemical has been cited by the EPA for violating the Clean Air Act, the Resource Conservation and Recovery Act, and the Clean Water Act. They have polluted the Tittawassee River, Saginaw River, and Saginaw Bay with extensive dioxin and furan contamination, resulting in a fine of 2.5 million dollars (EPA, 2011). Wacker Chemie suffered an accidental explosion in September of 2017 that injured several workers. Wacker Chemie was fined by the Tennessee Occupational Safety and Health Administration, for several serious violations, including the training of their employees, use of maintenance materials, and inspection of energy control (Wtvc, 2018).
Platinum mining takes place largely in South Africa, where it is not well regulated, and it causes a myriad of health problems. Platinum mining releases Sulfur Dioxide (SO2), which causes lung damage and aggravates heart disease, as well as “increased daily mortality” (Cairncross, 2004). It also releases particulate matter into the air where is again damages lungs, causes cancer, and leads to premature death, especially to the young, old, sick, or otherwise vulnerable. According to the WHO, there is no safe levels of either of these substances. Platinum mining is also environmentally horrific, as it takes 580,000 tons of ore per ton of platinum produced. It uses massive amounts of water and energy (while polluting that water) and releases incredible amounts of carbon dioxide (Cairncross, 2004).
Graphite mining takes place primarily in China, where it is again underregulated and causes a variety of environmental and health problems to people living in nearby villages. Not only does particulate matter in the air cause respiratory and heart problems, but the discharges from the mining contaminate local drinking water, making it non-potable. Local rivers do not freeze during the winter, and the water poisons nearby vegetation. The people living in these villages are too poor to move anywhere else, and are essentially stuck, living there against their will. Some companies that purchase large amount of graphite (typically smartphone manufacturers and lithium ion battery manufacturers) have stated that they are switching to synthetic graphite that is not mined, but whether they are or not and what effect that has remains unclear (Whoriskey, 2016).
Up until this point I have spoken mostly in general terms about chemical companies and mining in general. The problem with tracking a lot of this information down is that it isn’t readily available to the public but is regarded as a trade secret. I specifically did not focus on a fuel cell from a particular company, but even if I did, it is hard to find where specific pieces came from or what wastes come from combining them. I could not find anything on carbon paper or carbon fiber in this application and so decided to leave them out. It is apparent, however, that all these materials are being made or produced or mined in drastically different locations of the planet. That means you then have to transport them to wherever they are being assembled. China and other Asian countries have invested far more in fuel cells than the United States has, and so produces far more of them. These cars are mostly being purchased where there is infrastructure for them. This in in predominantly European counties, such as Sweden. In the United States, California has by far the most hydrogen refueling stations, and so leads the country in fuel cell consumption.
Meanwhile, there are problems with the hydrogen fuel itself. Hydrogen spills and hydrogen explosions don’t happen infrequently. The Wacker Chemie explosion from September of 2017 was a hydrogen explosion. One of the major drawbacks of hydrogen (and therefore some of the trepidation over driving on top of a tank of compressed hydrogen) is that it is explosive. But so is gasoline. And in fact, if you were so unfortunate as to get into an accident that ruptured the fuel tank (even though Toyota at least markets this as unlikely), gasoline would spill out and collect on the ground, whereas hydrogen would disperse into the atmosphere. This is a good thing for safety, but a bad thing for the planet. Hydrogen is a secondary greenhouse gas. When electrolysis and fuel cells are operating properly, there is no hydrogen leakage. Nevertheless, accidental spills and leaks do happen and would happen more if we used hydrogen more. “If a global hydrogen economy replaced the current fossil fuel-based energy system and exhibited a leakage rate of 1%, then it would produce a climate impact of .6% of the current fossil fuel based system” (Derwent, 2006).
Hydrogen not only acts as a greenhouse gas, but also as an ozone depleter. Ozone depleters reduce the amount of stratospheric ozone by reacting with the O3 molecule to turn it into a O2 molecule, which does not have the same capacity to protect against ultraviolet radiation. It is unclear how persistent hydrogen would be in the atmosphere, or how many ozone molecules it would destroy per hydrogen molecule released. The only other time we have really dealt with ozone depletion was in the 70s with CFCs. Part of the reason CFCs, or Chlorofluorocarbons, were so destructive is both their persistence in the stratosphere, and that one CFC molecule could destroy more than 100,000 ozone molecules per atom of chlorine, of which there are three per CFC molecule (Team, 2005). Some estimates put hydrogen ozone depletion at “8% more depletion over the North Pole, and up to 7% more over the South Pole” (Ball, 2003). However, this study estimates that hydrogen loss would optimistically be at 10%. There are solutions we can implement to bring this number down, such as making hydrogen fuel on site where fueling stations are and focusing on accident prevention with new technologies like self-driving cars. Also, while the ozone layer is still recovering from CFC emissions, it will have healed almost completely or at least a lot more by the time we get a hydrogen economy to be fully functioning (Ball, 2003).
There is not much information to be found on recycling and waste disposal of fuel cells. This technology is so new that it isn’t economically viable to recycle them. There isn’t much of value you can extract except for platinum. However, there is very little platinum in these fuel cells and more research is being done to become less dependent on platinum. Also, in most ways it is used, it is combined with other materials chemically. To separate them would require a chemical reaction. Unless recycling becomes much more efficient and economically worthwhile, most, if not all, of the fuel cell will go directly into the landfill, or some form of waste disposal. Some of the materials are quite toxic and would need special disposal.
Although fuel cells have some imperfections, given that we are staring down the barrel of climate change, it may be not only a viable option for the future, but a necessary one. While fuel cells can be used to power almost anything (given enough cells), they are currently being marketed as a replacement of the internal combustion engine for a personal automobile. But producing the car itself takes no more or less energy or materials or waste disposal than a typical car does to make. And that trend of everyone owning their own car, nevertheless driving that car, is one of the main problems. Fuel cells may give people an excuse to keep driving their cars, much in the same way electric cars have, even though our levels of consumption need to drop significantly if our resources are to sustain our growing population. Despite all of this, hydrogen fuel cells should not be easily dismissed. A significant problem with most alternative energies is their variability. The sun doesn’t always shine, the wind doesn’t always blow, so we need to build batteries to store that energy. The technology just isn’t there yet, and it’s a limiting factor. Fuel cells do not have that variability. You feed it hydrogen, it spits out energy and water. It is reliable and dependable, much like an internal combustion engine. Even if you don’t think climate change is a situation as dire as I have painted it to be, we are going to run out of fossil fuels in a century and a half. We need alternatives. And if we have any hope of stopping significant climate change, those fossil fuels need to be left in the ground. There are drawbacks to every form of energy. But fuel cells have drawbacks we can live with.
Sources:
Ball, Philip. “Hydrogen Fuel Could Widen Ozone Hole.” Nature News, Nature Publishing Group, 13 June 2003, www.nature.com/news/1998/030609/full/news030609-14.html.
Cairncross, Eugene. “Health and Environmental Impacts of Platinum Mining.” www.thejournalist.org.za/wp-content/uploads/2014/09/Environmental-health-impacts-of-platinum-mining1.pdf.
“Carbon Dioxide Concentration | NASA Global Climate Change.” NASA, NASA, 17 May 2017, climate.nasa.gov/vital-signs/carbon-dioxide/.
“Understanding 350.” 350.Org, archive.350.org/understanding-350.
Clabby, Catherine, et al. “GenX Pollution - What Happened? And When?” North Carolina Health News, North Carolina Health News, 18 Aug. 2017, www.northcarolinahealthnews.org/2017/08/17/genx-pollution-what-happened-when/.
Derwent, Richard & Simmonds, Peter & O'Doherty, Simon & Manning, Alistair & Collins, William & Stevenson, David. (2006). Global environmental impacts of the hydrogen economy. Int. J. Nuclear Hydrogen Production and Application Int. J. Nuclear Hydrogen Production and Application. 1. 57-67. 10.1504/IJNHPA.2006.009869.
“Dow Chemical Company Settlement.” EPA, Environmental Protection Agency, 6 Mar. 2017, www.epa.gov/enforcement/dow-chemical-company-settlement.
Hogue, Cheryl. “Chemours Told to Cut Fluorocarbon Air Pollution from North Carolina Plant.” CEN RSS, 27 Feb. 2018, cen.acs.org/articles/96/i10/Chemours-told-to-cut-fluorocarbon-air-pollution-from-North-Carolina-plant.html.
Kelly, Sharon. “DuPont's Deadly Deceit: The Decades-Long Cover-up behind the ‘World's Most Slippery Materia...” Salon, 5 Jan. 2016, www.salon.com/2016/01/04/teflons_toxic_legacy_partner/.
“Safety in Handling and Use.” Nafion™ Membranes - Delivering on the Promise of Clean Energy, Chemours Company, 2016, www.chemours.com/Nafion/en_US/index.html.
Team, ESRL Web. “ESRL Global Monitoring Division - Halocarbons and Other Atmospheric Trace Species.” NOAA Earth System Research Laboratory, 1 Oct. 2005, www.esrl.noaa.gov/gmd/hats/publictn/elkins/cfcs.html.
“Teflon and Perfluorooctanoic Acid (PFOA).” American Cancer Society, 5 Jan. 2016, www.cancer.org/cancer/cancer-causes/teflon-and-perfluorooctanoic-acid-pfoa.html.
“Toyota Mirai – The Turning Point.” 2018 Toyota Mirai Hydrogen Fuel Cell Vehicle | The Future of Everyday, 2018, ssl.toyota.com/mirai/fcv.html.
Whoriskey, Peter. “The Batteries in Your Favorite Devices Are Literally Covering Chinese Villages in Black Soot.” The Washington Post, WP Company, 2 Oct. 2016, www.washingtonpost.com/graphics/business/batteries/graphite-mining-pollution-in-china/.
Wtvc. “Wacker Chemie Cited, Fined Further after TOSHA Investigation into September Explosion.” WTVC, 7 Mar. 2018, newschannel9.com/news/local/wacker-chemie-cited-fined-further-after-tosha-investigation-into-september-explosion.