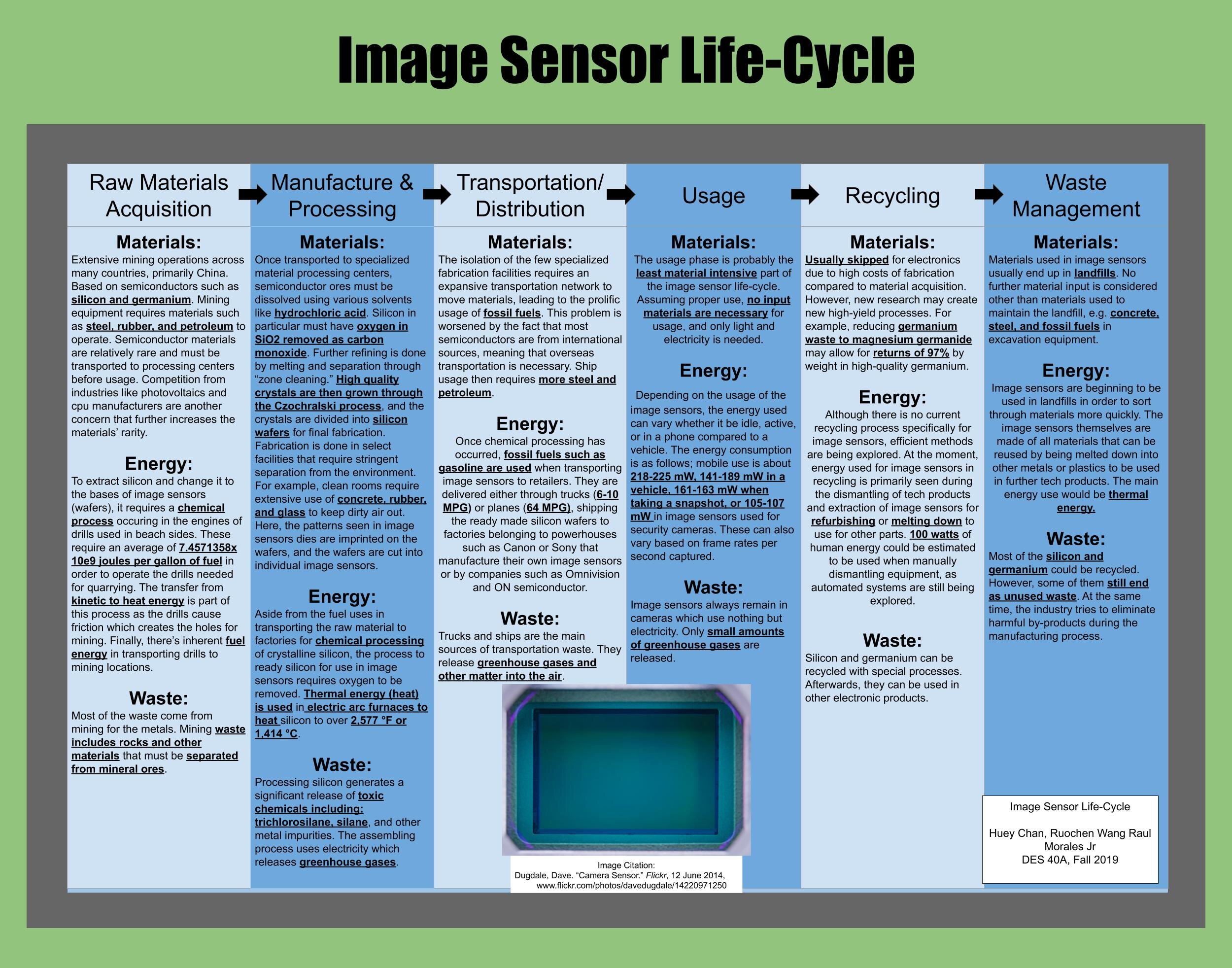
Design Life-Cycle
assess.design.(don't)consume
Materials
Huey Chan
Professor Cogdell
Design 40A
4 December 2019
Image Sensor Materials
One item most people use in their daily lives is the ubiquitous digital camera. As useful as it is, the digital camera would not be possible without the proliferation of reliable image sensors. Image sensors are semiconductor-based detectors that react to certain electromagnetic wavelengths to produce an electric output. For digital cameras, image sensors react to visible light to produce image data. The two most common types of image sensors are the complementary metal oxide semiconductor (CMOS) and the charge-coupled device (“Imaging Electronics”). Nevertheless, the focus will be on CMOS sensors. The fundamental building blocks of this type of sensor are metal-oxide-semiconductor (MOS) transistors and junction photodiodes (Guidash, et al.) These components are based on semiconductors such as silicon and germanium, two nonrenewable minerals that require extensive processes to use. Through attempts at fulfilling consumers’ insatiable appetite for better cameras, image sensor manufacturers require massive inputs of these and other nonrenewable materials; this analysis will discuss the usually negative environmental effects of the raw materials used and the effects of materials seen in the subsequent processing, manufacture, distribution, use, and disposal from obsolescence.
The materials used in life-cycles are important in understanding the environmental impact of image sensors. Since there may be numerous variations in the materials and methods used to construct image sensors, the following life-cycle steps are only examples. The cycle begins with the initial extraction of raw materials. Refined silicon and germanium are not primary raw materials, since they must be created from other materials extracted from the Earth. The extraction of the primary raw materials is a global industry, with numerous mining locations in different countries, especially China (Decker). Silicon in particular may be mined in the form of high-purity quartz (“Wafer Fabrication”). This process alone requires several input materials, such as metals in the mining equipment and petroleum to power them. There are various other sources of silicon, such as silica, but they must all be ultra-pure to be used in electronics (“Silica”). These mining operations often have a harmful effect on the environment, usually requiring the site to be cleared of flora and fauna. Once the silicon ore is mined, the natural oxygen present in the silicon (SiO2) must be removed through melting the quartz in furnaces. The quartz is heated up to 1,460 °C, and the oxygen is released in the atmosphere as carbon monoxide, a toxic gas (“Wafer Fabrication”). The material requirement of this step is variable, but it is minimal outside the materials used the construct the furnace and the purging gases, assuming the furnace is electric. Once free of oxygen, the resulting silicon is still too impure to be directly used. The next step is distillation using hydrochloric acid to produce hydrogen gas and a chemical known as trichlorosilane. This process is then reversed to create purer silicon, but further refinement is still required for use in electronics. A process known as “zone cleaning” uses high frequency coils to melt the silicon into ingots and refine it until it is 99.9999999 pure, or less than 1 part per billion in foreign particles (“Wafer Fabrication”). After all of these material and energy-intensive activities, the silicon is finally useful for image sensors. The various solvents and acids mentioned must also be carefully handled to prevent harmful effluence into the environment. For germanium, the rarity of this material compared to silicon means its sparing usage in image sensors. The most common sources of germanium include iron meteorites, terrestrial iron-nickel, sulfide ore deposits, iron oxide deposits, pegmatites, greisens, skams, coal, and lignitized wood. Overall, these germanium sources usually have an average concentration of several hundred ppm or less (Bernstein 2409). The mining procedures needed must be similarly material and energy-intensive compared to silicon mining. Also like silicon, germanium ore must be extensively processed using similar methods of refinement. This begins with separating impurities with hydrochloric acid, similar to silicon. The output of germanium tetrachloride is distilled and hydrolyzed to form germanium dioxide. Hydrogen is then used to reduce the germanium dioxide to a powdery metal, which can be melted at around 1,100 °C to form ingots of germanium suitable for electronics (“Germanium”). The chemicals and heat used come with similar environmental concerns as silicon.
With the refining complete, the transportation to specialized fabrication facilities is the next step. The stringent requirements of fabrication facilities, such as needing a clean room, mean that there are only a select few locations where fabrication can take place. This reality exacerbates the environmental costs of transportation, with most methods requiring fossil fuels. Once the semiconductor ingots reach a fabrication facility, an operation known as the Czochralski process grows single crystals from the ingots, and the resulting crystalline cylinder is cut into thin, flat shapes called “wafers” (“Semiconductor”). Various processes are then used to create the necessary layers on the wafers for image sensors. These processes number in the hundreds, but they can be categorized as deposition, removal, electrical modification, or patterning (“Semiconductor”). The creation of wafers can take several months, and fabrication companies seldom release information on the specifics of the processes, especially the required materials. After the wafers are completed, they are cut into pieces that will become individual image sensors. The number of sensors per wafer varies depending on the sensor die size, and potential defects in the wafer will reduce the total yield (“Understanding”). The potential for defects is a common problem across wafer processing, and it inevitably increases the material costs of precious semiconductors. The material costs in creating the necessary clean rooms must also be considered, with the prolific usage of concrete, metals, plastics, rubbers, and glass in both the air-tight buildings and ventilation systems. Still, the cut and accepted dies are finally ready for use as image sensors.
Once more, transportation is necessary to bring the completed image sensors to digital camera manufacturers. The sensors are then assembled into the cameras, with their inherent usage of various plastics, metals, and glass. The manufacture of cameras is highly varied, with the details in construction and layout dependent on intended use and aesthetic considerations (“Understanding”). Of course, transportation is required to bring the camera to consumers for use, and this leads to the life-cycle stage that most people are familiar with. This stage has virtually no material considerations compared to the most material and energy-intensive early stages. Other than light and minimal electric requirements, little maintenance is needed for the usage of image sensors (“Imaging Electronics”). The electronic nature of the sensors also preclude most chances of emissions. Due to the rapid advancement of camera technology, the usage of image sensors usually ranges from a few months to no more than a few years. After usage is complete, the camera along with the image sensor is often simply disposed of, with little regard for extended use or recycling.
To elaborate, the final parts in the image sensor life-cycle involve the final disposal of image sensors. Although the scarcity of semiconductors would encourage efforts at material recovery, the current disproportionately large cost of image sensor fabrication compared to mining reduces the desirability of recycling. Industrial leaders simply do not consider the returns of recycling worthwhile in offsetting the costs of production (Decker). The result of this attitude is that image sensors, like most electronic waste, end up in landfills. Due to the life-cycle of image sensors simply ceasing in this manner, the whole process of mining, manufacturing, transportation, use, and disposal must begin again with new primary raw materials. This problem is only worsened with the increasingly shorter lifespans and quick obsolescence of electronics (Decker). With the earlier mentioned damages repeating, the unsustainability seen in the image sensor business will cause a perpetual cycle of environmental devastation.
However, new research is being conducted that may alleviate the devastation at least somewhat. There is a hesitation to conduct recycling of semiconductor products, but the promises of high-yield returns from some new processes may overcome such attitudes. An example of this new research include procedures to convert the semiconductor waste into mixtures of magnesium germanide and magnesium silicide, allowing the return of ultra-pure material. The purported yields for the rarer germanium are up 97% weight in returns (Bumba, et al. 8859-8860). Efforts such as this will become increasingly enticing as stocks of primary raw materials diminish and material competition from semiconductor industries increase. The manufacture of photovoltaics is a clear example of competition for image sensors, where photovoltaics used 3,400-4,600 tons of the silicon feedstock annually (Sarti and Einhaus 30). With all semiconductor industries expanding, mining and refining operations may not be sufficient to meet demand (Joch). Recycling semiconductors may be the only strategy that can allow for the continued growth of image sensors without environmental destruction.
Going through this example life-cycle for image sensors, several concessions had to be made regarding information available in the public. In addition, several assumptions were necessary to ensure the cycle’s completeness. For example, the exact procedures for fabricating image sensors on silicon wafers were impossible to obtain using online sources due to such information being trade secrets. The little available information on the processes stated that there “may be more than 250 very high technology steps required to manufacture a semiconductor” (“Semiconductor”). Furthermore, several cut-offs had to be decided on the needed materials for the life-cycle. The materials in mining equipment were considered, but the materials in the equipment that made the mining equipment were left out for example. Also, it was assumed that mining and initial processing occurred in the same area and the same form transportation was used for all life-cycle stages. With these limitations, this life-cycle analysis only offers a general summary of the environmental effects of image sensor materials.
Mining and manufacturing, especially the manufacturing, possess the lion’s share of embodied energy for image sensors. The massive material costs of these beginning processes are only worsened with the shortening life-cycles and outright disposal of electronics, leading to a repetition of the harmful processes (Decker). When looking at the impact of image sensors, the energy and materials used by consumers are only a small fraction of the total embodied energy of this product. The whole life-cycle must be considered when deciding how to improve sustainability. Research into more efficient fabrication and more economical recycling may be the only viable method to avoid this perpetual cycle of environmental devastation from consumer demand.
Bibliography
Bernstein, Lawrence R. “Germanium Geochemistry and Mineralogy.” Geochimica Et Cosmochimica Acta, vol. 49, no. 11, Nov. 1985, pp. 2409–2422, doi:10.1016/0016-7037(85)90241-8.
Bumba, Jakub, et al. “Total Germanium Recycling from Electronic and Optical Waste.” Industrial & Engineering Chemistry Research, vol. 57, no. 27, 11 July 2018, pp. 8855–8862, doi:10.1021/acs.iecr.8b01237.
Decker, Kris De. “The Monster Footprint of Digital Technology.” Edited by Vincent Grosjean, Low-Tech Magazine, 16 June 2009, www.lowtechmagazine.com/2009/06/embodied-energy-of-digital-technology.html.
Gabrys, Jennifer. “The Quick and the Dirty: Ephemeral Systems in Silicon Valley.” Thresholds, no. 31, 2006, pp. 26–31. JSTOR, www.jstor.org/stable/43876268.
“Germanium.” Edited by Adam Augustyn, Encyclopædia Britannica, www.britannica.com/science/germanium.
Guidash, R. M., et al. “A 0.6 Μm CMOS Pinned Photodiode Color Imager Technology.” International Electron Devices Meeting. IEDM Technical Digest, doi:10.1109/iedm.1997.650533.
“Imaging Electronics 101: Understanding Camera Sensors for Machine Vision Applications.” Edmund Optics Worldwide, www.edmundoptics.com/resources/application-notes/imaging/understanding-camera-sensors-for-machine-vision-applications/.
Joch, Alan. “Sand Trap.” Plenty Magazine, 10 Nov. 2006, www.plentymag.com/features/2006/11/sand_trap.php.
McCracken, Katherine E., and Jeong-Yeol Yoon. “Recent Approaches for Optical Smartphone Sensing in Resource-Limited Settings: a Brief Review.” Analytical Methods, no. 36, 2016, pp. 6591–6601, doi:10.1039/C6AY01575A.
Sarti, Dominique, and Roland Einhaus. “Silicon Feedstock for the Multi-Crystalline Photovoltaic Industry.” Solar Energy Materials and Solar Cells, vol. 72, no. 1-4, Apr. 2002, pp. 27–40, doi:10.1016/s0927-0248(01)00147-7.
“Semiconductor Manufacturing and Fabrication Review.” Engineers Edge, www.engineersedge.com/manufacturing/semiconductor_fabrication.htm.
“Silica.” Minerals Education Coalition, www.mineralseducationcoalition.org/minerals-database/silica/.
“Understanding The Digital Image Sensor.” LUCID Vision Labs, www.thinklucid.com/tech-briefs/understanding-digital-image-sensors/.
“Wafer Fabrication: Production of Raw Silicon.” Halbleiter.org, www.halbleiter.org/en/waferfabrication/rawsilicon/.
Embedded Energy
Raul Morales Jr Morales 1
Design 40A
Group Members: Huey Chan, Ruoacheng (Max) Wang
4 December 2019
Energy in an Image Sensors Life
Image sensors are one of the most used pieces of new technology to come out in the last decade. Used at traffic lights, in automobiles, phones, cameras, etc, image sensors serve a universal use and are used by everyone, even if they don’t know it. But passes by without notice is the energy used within the process of creating such a small piece of tech that makes every digital or that contains an image possible. The energy used to extract raw material such as silicon, to process it and ultimately prepare it for use in image sensors, along with out essentials, is a step by step process that embodies all types of energy from thermal to mechanical, chemical and electrical. The multi-step process to creating such an important piece of technology is universal and travels across seas all around the world to be able to allow consumers to take a picture, drive safely, and ultimately see the world differently through the smallest means of material use.
The extraction of raw material for the production of image sensors, more specifically, Silicon, is a high energy consuming procedure that uses fossil fuels and chemical and thermal energy to purify Silicon from sand. The main basis for the production of Image Sensors is silicon which is primarily extracted from sand, but not just any sand, it must be silica based sand that contains quartz crystal which carry the silica compound (Bieser). In order to obtain the quartz, manufacturers must follow a process called quarrying. Quarrying requires a drilling rig which primary run on diesel engines and uses about 62 to 83 gallons of diesel fuel which roughly averages about 1,500 gallons of diesel fuel a day, with every “U.S. gallon of gasoline containing 114,000 BTUs of energy, or about 120 million joules,” (Woodford). Explosives are then put into the holes that have been made that explode about 60,000 tons of rock. Once the rock is extracted dump trucks carry the rock into crusher, which uses mechanical energy using about 800 kw to 1200 kw in order to successful crusher the rock, a process which is then repeated twice for rock formations that need to be crushed again.
Although the extraction of silica is an extensive process in itself, the purification of silicon requires a variety of energy sources, which include mechanical and chemical and thermal energy in order to melt the raw material and eventually cut and buffer the silicon wafers for distribution (Edmund Optics). The lengthy and time consuming process of turning silica from quartz into polysilicon and final into silicon wafers starts with quartz being put into an electric arc furnace that is heated to 2,000 °C powered by a three face electrical supply averaging about 300 to 400 kwh per electric ton. In this furnace which, a chemical process occurs in which the quartz is reduced with coke to produce metallurgical grade silica. Distillation takes about 3-4 steps in which the newly made material from metallurgical grade silica to triclorcyclede which is boiled at 31 °C. The hyperbolic trichloroethylene is reduced by hydrogen at 1100 °C forming silicon and deposited by rods in which they grow; an overall process that requires extensive heat and chemical reactions, melting, boiling, and distilling raw material to form pure polysilicon which is finally crushed into chunks and graded. (Chemistry AG).
Once the raw polysilicon is made, it is transported to another manufacturer that specializes in breaking down polysilicon to be used to form silicon wafers. The polysilicon is heated to 2500°F in a sealed furnace that uses joule heating which passes an electric current through the conductor to produce heated, more specifically approximately 100 to 200 kw of energy. Once the liquid is spun and turn into a large solid crystal, it is checked with chemicals and x rays which uses approximately 100 eV to 200 keV. The crystal is then sliced using kinetic energy to move a filigree wire at a speed of 60 km/h. This is done for an approximate length of six hours, cutting wafers of silicon that are about 180 µm thick. (Fraunhofer-Gesellschaft). The wafer is then sent through lapping, which is a buffing process that … Finally, as the silicon wafers are being finished, the room they are made in must be cleared of all possible dust that can affect the wafer (Delair). 12,000 tons of air conditioning equipment is used to purify the air. A 3-ton unit of air conditioning would use about 36,000 BTU’s of energy or 12,000 BTU’s per unit. Therefore, in order to purify the air of dust, silicon manufacturers use approximately 144,000,000 BTU of energy (Discovery Science).
Once the wafer is ready, the next steps are to create the glass and ceramic. Creating the ceramic casing for semiconductors is a multi step process that requires the chemical processing of different materials to create the ceramic used in the casing. CHemical energy is used when mixing these products using automated systems running on electrical power using AC power units, with an average AC unit using approximately 3,000 to 5,000 watts of energy. A similar process occurs when creating the light transparent glass used to protect silicon wafer.
Once the Image sensors are ready they are transported to some of the leading companies that utilize the image sensor such as Sony, Omnivision, Canon, etc. SOme distribution methods to facilities such as Sony factories in Japan or China requires flight transportation to other distributors or small retailers. An average plane will use approximately 1 gallon of gas every second, with a duration of flights across seas being over 10 hours some of the transporting of image sensors on plane can amount to approximately 36,000 plus gallons of fuel, estimating about 4.3299498 x 1012 Joules. Many manufacturing facilities are held in the content of Asia with some of the major headquarters of these business being in America. This involves another set of transportation from planes to trucks in order to transport materials to retailers. Fossil fuels such as gasoline are used when transporting image sensors to retailers by trucks which estimate to use roughly 6-10 MPG.
Image sensors are consistently being used in almost every technological product from automobiles to phones and cameras. Even when the devices are not in use and the image sensors are in an idle state they still consume a lot of energy. In mobile phones that are not in use the image sensors uses up about 218 to 225 mW of energy and when it is active takes up about 225 to 338 mW. In automobiles where image sensors are uses an exterior sensors to detect when there are vehicles or object near the vehicle they use about 141.8 mW and when active they use 189.5 mW. Finally in cameras, when not in use the image sensors uses about 161.9 mW and when activated it uses about 163.5 mW. Maintaining these sensors as clean and polished so they will function properly requires manual labor exerting more than 100 watts of human energy.
Due to the influx of image sensors being made consistently and the cost of mining for the initial raw material as opposed to the output of recycling such a small amount of silicon and material in image sensors, there is no set recycling method used to properly dispose of image sensors. There is also a difficulty in tracing the status of reused recycled products of this scale. There are many companies however such as APTO Solution that will recycle electronic products by breaking them down materials and separating them. \Magnets, storing about 80 Joules, are used to separate metals from circuit boards. The circuit boards are then stripped as well or can be refurbished in order to be used in further electronics. Some electronic companies have their own recycling programs in which they will take in their own products and break them down with a similar process as APTO in order to strip the parts that can be used from those that are a waste.
The majority of electronics are not properly recycled and end up as E-waste creating landfills outside some of the manufacturing companies in India or China. HUman labor is used to dismantle and burn products, using 75-150 watts of human power during a regular 8 hour work shift. With an estimated 60,000 workers working to dismantle products such as those in China, energy used amounts a maximum of 9,000,000 watts of energy used. This is only half the battle as the majority of e waste sites in landfills and hurts the environment which in itself causes more energy use when trying to save animal and plant populations.
Ultimately, the process of creating what has become an essential product in our lives takes a never ending process of chemical and thermal energy to purify and break down crystals and elements along with mechanical and electrical energy to create the wafers that are the basis for every image sensor made. Automated machines as well as automobiles all consume large amounts of energy to transport these materials to distributors and companies that use even more chemical energy to distribute to retailers. Since image sensors are in such high demand there seems to be no end in their development, which calls for smarter recycling and waste methods for said products. At the moment since the cost of extraction to even make this products surpasess cost of properly recycliong these materials, we see a never ending expanse of consumer without the knowledge of how to dispose of their electronics and ultimately increasing the e-waste as more image sensors and electronic products are being made that require said sensors. The backbone of the entire process bieng the earth itself, that seems to be reaping the consequences of the new era.
Citations
Beiser, Vince. “The Ultra-Pure, Super-Secret Sand That Makes Your Phone Possible.” Wired, Conde Nast, 30 Oct. 2018, https://www.wired.com/story/book-excerpt-science-of-ultra-pure-silicon/.
Woodford. “Drilling Science and Technology.” Explain That Stuff, 16 Nov. 2018, https://www.explainthatstuff.com/drilling.html.
Haque, Tajirul. “Introduction to Electronics Recycling.” The Balance Small Business, The Balance Small Business, 7 Oct. 2019, https://www.thebalancesmb.com/introduction-to-electronics-e-waste-recycling-4049386.
“Imaging Electronics 101: Understanding Camera Sensors for Machine Vision Applications.” Edmund Optics Worldwide, Edmund Optics Worldwide, https://www.edmundoptics.com/resources/application-notes/imaging/understanding-camera-sensors-for-machine-vision-applications/#construction.
How Much Fuel Does an International Plane Use For a Trip?
HowStuffWorks.com Contributors - https://science.howstuffworks.com/transport/flight/modern/question192.htm
“How Sand Is Transformed into Silicon Chips.” HPQ Materials, 3 June 2019, www.hpquartz.com/2009/05/24/how-sand-is-transformed-into-silicon-chips/.
New Media Ltd. Quarrying Process And Quarry Products, https://www.northstonematerials.com/quarrying_process_and_quarry_products.
“Slicing Silicon Blocks Into Paper-Thin Wafers For Solar Cells.” ScienceDaily, ScienceDaily, 7 Aug. 2009, https://www.sciencedaily.com/releases/2009/08/090807103917.htm.
“Silicon.” How Products Are Made, http://www.madehow.com/Volume-6/Silicon.html.
“Our HVAC Blog: Del-Air Heating & Air Conditioning.” Del, 9 Apr. 2019, https://www.delair.com/blog/florida-air-conditioning-costs-tips-to-lower-your-bill-during-the-summer-heat.
Peters, Adele. “See Inside The Hellish E-Waste Dumps Where Old Electronics Go To Die.” Fast Company, Fast Company, 17 Jan. 2018, https://www.fastcompany.com/40515861/see-inside-the-hellish-e-waste-dumps-where-your-old-electronics-go-to-die.
Waste and Pollution
Ruochen (Max) Wang
DES 040A/SAS 043 A02
Group members: Huey Chan, Raul Morales
Image sensors
12/4/2019
Waste in the lifecycle of the image sensor
The image sensor is sensors that detect and transmit information used to make an image. It does it by converting the light waves into signals that transmit the information (LUCID). Building an image sensor, which is a transistor, requires high precision and purification process in each stage of the manufacturing process. Each step in the process produces high amounts of waste and emissions, so the amount of waste produces in their life cycle is enormous. Therefore it is imperative to consider the waste produce and waste management of all the waste produced in the lifecycle of an image sensor. Most importantly, the waste generated when building the sensor and all silicon and germanium that got thrown away after a sensor is not working anymore.
The first stage of building an image sensor is obtaining the pure silicon wafer, which is the base material for the sensor. The two main materials used to make silicon wafers are silicon and arsenic. However, pure silicon cannot found naturally in nature, so people use quartz as the primary source to get silicon (Hughes). People can find quartz in rocks like sandstone, gneiss, or granite. People get it by open pit mining using big excavation machines. Due to low concentrations of minerals needed in the ore, it generates large amounts of solid waste. Waste rock or materials that cover the metal or mineral body are thrown away when the valuable parts are separated from the part that is not going to be used (Nagaraj). Those solid waste are called tails, and they are usually disposed into tailing ponds, which is a wet storage area for the solid residues (CBC News). Tailing ponds need to be designed correctly in order to store mining wastes indefinitely. Once a tailing pond is full, the worker can drain the water, and the process poses no risk (CBC News). However, sometimes, solid waste is disposed of at low costs to save money, but this causes further damage to the environment. There is a high possibility of leaving the waste rocks in the mines after finished mining or to nearby canyons. That leads to extensive corrosion of the soil, which dry the ground and make the soil loss nutrients. That can cause desertification of the surrounding area. The physical changes in the environment with chemical leak and displacement of surrounding sediment and minerals in the water can poison and destroy life around the area. The increase in particle in the water due to leak may lower the water quality or poison it. Also, the dust has a significant effect on humans, crops, and animals in the area. It can cause some severe illnesses and diseases (Menezes).
After getting the quartz, it needs to undergo different purification process which requires different chemicals and produces toxic chemical byproducts. The first step, heat the quartz to over 2000 degrees Celsius with the presence of carbon to cause the carbon dioxide emissions to extract out the oxygen from the silica and leave only silicon behind. They are then treated with oxygen to get rid of calcium and aluminum impurities, which resulting in metallurgic-grade silicon (~99% pure). However, it needs to be 99.999999% pure silicon, so it is further treated with gaseous hydrogen chloride (HPQ Materials). The reaction produces trichlorosilane gas; it contains many chlorinated metal impurities such as iron, aluminum, or carbon (Mulvaney). These impurities are removed by distillation and become waste products. Then add heat and hydrogen to transform it into silane gas, which is environmental and health hazard. Later, it is deposited on an ultra-pure silicon rod to result in electronic-grade silicon. After getting the pure silicon, the silicon wafer can be manufactured. For every one eighth inch silicon wafer made, there are 3787 gallons of wastewater, 27 pounds of chemical, and 29 cubic feet of hazardous gas wastes are produced (Holden).
The next stage is to processed germanium, which is used in making the glass part of the sensor. Just like silicon, germanium is not found naturally in nature; it is produced as a byproduct of base metal refining. Germanium is commonly produced from sphalerite zinc ores, and it is also known to be extracted from fly ash coal and some copper ores. No matter what source of material it comes from, germanium is first purified using chlorination and distillation process to produce germanium tetrachloride (Ruiz). Distillation removes impurities, and those impurities become waste products. Then germanium tetrachloride is hydrolyzed and dried, which produces germanium dioxide. After that, the oxide is reduced with hydrogen to form germanium metal powder (Ruiz). During those processes, the byproducts become wastes. Later, cast the germanium powder into bars at temperatures over 938.25 degrees Celsius. After getting the powder into the bar, zone-refining is used to removes impurities and produce high purity germanium bars (Bell). That germanium is often more than 99.999% pure. Then, zone-refined germanium is further be grown into crystals, which are sliced into thin pieces for use optical lenses (Bell). This process produces germanium dust, but it is relatively non-toxic; however, in some situations, it gives immediate hazards to personnel or the environment (Lattice material). The overall process does produce a large number of side products that are chemical wastes, but most of them are relatively non-toxic. At the same time, semiconductor industries are trying to eliminate the amount of harmful chemical residues produces in the purifying process by using different process methods and less toxic chemicals.
After getting all the material needed to build a sensor, different pieces are put together to make the sensor in the factory. During all those processes from manufacture the raw material to put all pieces together, electricity is the primary power source and waste source. There are various ways to produce electricity, like using nuclear power, natural gas, coal, etc. At the same time, people are trying to use more renewable energy sources. However, the primary source of electricity generation is still burning fossil fuel. As a result, burning fossil fuel, like coal, releases nitrogen oxides, carbon monoxide, and other greenhouse gases. Since modern society sill heavily depends on electricity, to decrease the negative environmental effect of electricity production, people need to find ways to have better use of renewable energy that can replace nonrenewable energy.
Not only the manufacture and processing of material to build products produce wastes, transporting those raw materials and final product also provide a large number of residues. Since silicon and germanium are from sources all over the world, oversea transportations are necessary. In this case, all kinds of transpiration are used like ships, trains, tracks, and sometimes planes are also used. While transporting on the ship, tracks, or railroads, greenhouse gases such as carbon dioxide, carbon monoxide, and nitrogen oxides are released (LIPASTO). If used a plane for transportation, they use a particular type of fuel called jet fuel, which is a mixture of a large number of different hydrocarbons (ICAO). In the flight, aircraft engines emit heat, noise, particulates, and all kinds of gases. For the chemicals, it emitted like carbon dioxide, water vapor, hydrocarbons, carbon monoxide, nitrogen oxides, sulfur oxides, and black carbon all interact among themselves and atmosphere, which causes the greenhouse effect. According to a study, 113 grams of carbon dioxide are released every one kilometer of the plane travels (LIPASTO). Since global warming still a big concern, people are trying to reduce greenhouse gas emissions. To lower greenhouse gas emissions, many car companies have built hybrid vehicles. Even though most of those companies are aiming at everyday consumers, such technology is slow to apply to current methods of shipping and distribution.
On the other hand, just like everything else that uses electricity, using the image sensor in the camera is going to release nitrogen oxides, carbon monoxide, and other greenhouse gases into the air due to electricity generation through fossil fuel. However, since an image sensor uses a minimal amount of electricity, the environmental impact it causes is minimal.
They will stop working someday in the future, and some metal materials that they are made of, such as silicon and germanium, will get recycled or disposed of just like other electronic components. However, many of them still end up in waste; for those, there are new ways found in recent years to make them recyclable. One method dealing with silicon waste is put the waste silicon sludge into a container and use acid treatment to remove impurity. Then let silicon undergo ultrasonic dispersion in an ultrasonic atomizer. After that, let it undergo ultrasonic spraying. Later dry it to get silicon powder, which are materials that can make Lithium batteries (Huang). All the impurity removed can also be recycled and transform into useful content in other areas. Another method dealing with silicon waste, also work with germanium waste. People can react silicon or germanium scrap with Magnesium scrap to created Magnesium germanide/silicide. Then undergoes hydrides to get Ultra-pure semiconductor and pure hydrogen (Bumba). That process not only returns the waste silicon and germanium to pure semiconductor but also produce zero waste, all the products are useful in different industries. However, since those processes take a lot of energy, it is not widely used.
There is also another electronic waste, such as other minor toxic substances (Wath). Releasing these substances into the environment would be hazardous, and nowadays, we do have the technology to recycle them. Advanced Technology Materials Inc. (ATMI) has developed a selective chemical process, which is hydrometallurgical processing. The process recovers valuable materials from electronics using a “green chemistry” technology. It is cost-effective and does not require shredding or grinding. Also, it is environmentally safe, and most importantly, it reduces the loss of precious metals. However, it does require the electronics to be separated from other everyday wastes (Baeyens, Lettieri, and Salem).
Nowadays, in 2019, we do have the tools to recycle non-environmental friendly materials within a transistor. However, it also often requires the awareness of consumers as they need to recycle their electronics at specific locations (Namias). Even though it is a tough task, but as more and more people realize how important it is to protect our precious environment, they know the importance of recycling and start moving towards a sustainable life.
Bibliography
Al-Salem, S.M., et al. “Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review.” Waste Management, Pergamon, 3 July 2009, www.sciencedirect.com/science/article/pii/S0956053X09002190.
Bell, Terence. “Get Information on the Properties and Applications of Germanium.” The Balance, The Balance, 25 June 2019, www.thebalance.com/metal-profile-germanium-2340135.
Bumba, Jakub, et al. “Total Germanium Recycling from Electronic and Optical Waste.” Industrial & Engineering Chemistry Research, Industrial & Engineering Chemistry Research, 7 June 2018, https://pubs.acs.org/doi/10.1021/acs.iecr.8b01237.
CBC New. “Tailings Ponds: What's in Them and What Risks Do They Pose? | CBC News.” CBCnews, CBC/Radio Canada, 11 Aug. 2014, www.cbc.ca/news/technology/tailings-ponds-for-mining-and-oilsands-waste-faqs-1.2727889.
Earl, David A. EPA, Environmental Protection Agency, 6 Dec. 2007, https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/8693/report/F.
FPFIS. “Analysis of Material Recovery from Silicon Photovoltaic Panels.” EU Science Hub - European Commission, EU Science Hub - European Commission, 26 July 2019, https://ec.europa.eu/jrc/en/publication/analysis-material-recovery-silicon-photovoltaic-panels.
“Harmful Effects of e-Waste Dumping.” GreenCitizen, GreenCitizen, https://greencitizen.com/learn-more/harmful-effects/.
Holden, Jason, and Christopher Kelty. “The Environmental Impact of the Manufacturing of Seminconductors .” OpenStax CNX, 2005, cnx.org/contents/Sc3-Pqne@4.35:7238FjUe@3/The-Environmental-Impact-of-the-Manufacturing-of-Seminconductors-ticket-5622.
“How Sand Is Transformed into Silicon Chips.” HPQ Materials, 3 June 2019, www.hpquartz.com/2009/05/24/how-sand-is-transformed-into-silicon-chips/.
Huang, Jiaxing. “From Silicon Waste to Batteries.” Jiaxing Huang Group, jxhuang.mccormick.northwestern.edu/discoveries-and-stories/better-living/waste-to-batteries/.
Hughes, Emma. “High Purity Quartz: a Cut Above.” Industrial Minerals, 25 Nov. 2013, www.indmin.com/Article/3282450/Magnesia-Features/High-purity-quartz-a-cut-above.html.
ICAO. “ICAO Global Framework for Aviation Alternative Fuels // .” ICAO Global Framework for Aviation Alternative Fuels, www.icao.int/environmental-protection/GFAAF/Pages/default.aspx.
“Imaging Electronics 101: Understanding Camera Sensors for Machine Vision Applications.” Edmund Optics Worldwide, Edmund Optics Worldwide, https://www.edmundoptics.com/resources/application-notes/imaging/understanding-camera-sensors-for-machine-vision-applications/#construction.
Katherine E. McCracken, Jeong-Yeol Yoon. “Recent Approaches for Optical Smartphone Sensing in Resource-Limited Settings: a Brief Review.” Analytical Methods, The Royal Society of Chemistry, 11 Aug. 2016, https://pubs.rsc.org/en/content/articlelanding/2016/ay/c6ay01575a#!
Lattice material. “Germanium Metal Material Safety Data Sheet.” Lattice Material, 24 Apr. 2003, www.sydor.com/wp-content/uploads/2019/05/Germanium-MSDS-1.pdf.
LIPASTO. “Unit Emissions - Freight Transport - Air Traffic.” LIPASTO, 2009, lipasto.vtt.fi/yksikkopaastot/tunnusluvut/tunnusluvutilmae_t.htm.
Mcallister, Lucy. “The Human and Environmental Effects of E-Waste.” Population Reference Bureau, Population Reference Bureau, 4 Apr. 2013, https://www.prb.org/e-waste/.
Menezes, Romualdo Rodrigues, et al. “Recycling of Mine Wastes as Ceramic Raw Materials: An Alternative to Avoid Environmental Contamination.” IntechOpen, IntechOpen, 29 Feb. 2012, www.intechopen.com/books/environmental-contamination/recycling-of-mine-wastes-as-ceramic-raw-materials-an-alternative-to-avoid-environmental-contaminatio.
Mulvaney, Dustin. “Hazardous Materials Used in Silicon PV Cell Production: A Primer.” Hazardous Materials Used in Silicon PV Cell Production: A Primer., Solar Industry Magazine, 2013, www.solarindustrymag.com/online/issues/SI1309/FEAT_05_Hazardous_Materials_Used_In_Silicon_PV_Cell_Production_A_Primer.html.
Nagaraj, D. R. “Minerals Recovery and Processing.” Wiley Online Library, American Cancer Society, 2 Dec. 2005, onlinelibrary.wiley.com/doi/abs/10.1002/0471238961.1309140514010701.a01.pub2.
Namias, Jennifer. “THE FUTURE OF ELECTRONIC WASTE RECYCLING IN THE UNITED STATES: Obstacles and Domestic Solutions .” THE FUTURE OF ELECTRONIC WASTE RECYCLING IN THE UNITED STATES: Obstacles and Domestic Solutions , July 2013, www.seas.columbia.edu/earth/wtert/sofos/Namias_Thesis_07-08-13.pdf.
Ruiz, Aixa González, et al. “Germanium: Current and Novel Recovery Processes.” IntechOpen, IntechOpen, 3 Oct. 2018, www.intechopen.com/books/advanced-material-and-device-applications-with-germanium/germanium-current-and-novel-recovery-processes.
Sarti, Dominique, and Roland Einhaus. “Silicon Feedstock for the Multi-Crystalline Photovoltaic Industry.” Solar Energy Materials and Solar Cells, North-Holland, 27 Nov. 2001, https://www.sciencedirect.com/science/article/pii/S0927024801001477.
Saurabh. “What Is a MOSFET?: Basics, Working Principle & Applications.” Electronics For You, 9 Oct. 2019, https://electronicsforu.com/resources/learn-electronics/mosfet-basics-working-applications.
Wath, Sushant B., et al. “E-Waste Scenario in India, Its Management and Implications.” SpringerLink, Springer Netherlands, 12 Feb. 2010, link.springer.com/article/10.1007/s10661-010-1331-9.
“Understanding The Digital Image Sensor.” LUCID Vision Labs, LUCID Vision Labs, https://thinklucid.com/tech-briefs/understanding-digital-image-sensors/.