Design Life-Cycle
assess.design.(don't)consume
Daria Zadorozhnaya
DES 40A
Professor Cogdell
December 4, 2018
The Rubik’s Cube: Puzzle of the Mind and of the Material
From the time of its creation in the 1970s by the Hungarian university professor Erno Rubik, the aptly named Rubik’s Cube has since delighted, confused, and presented an intricate puzzle that has both baffled and offered challenge to many minds. As a beloved toy, the Rubik’s Cube appears simple at first glance, seemingly made of only moveable plastic parts and a few stickers. However, like most objects of the modern world, the day-to-day items that most overlook as being simple and made of basic, rudimentary parts actually conceal multitudes of complex processes involving raw materials, all of which have their own properties, origins, effects on the environment, and contribution to the product as a whole. Although it’s tempting to write off the Rubik’s Cube as being made only of simple plastic, the following paper will explore the many different components involved in the manufacturing of the product, examining in depth the complex chemical ingredients and raw materials, their origins, their processes of extraction, and what roles they play from the beginning to the end of the life cycle.
The Rubik’s Cube is composed of three primary parts, the first of which is the most commonly recognized ingredient, and the one that makes up the majority of the product: the plastic. The overall structure of the puzzle is built of this, comprising of twenty-six cubes -some fixed and some mobile- that are all fastened to a central core [1]. The plastics that are used in the production of Rubik’s Cubes are of the variety known as thermoplastics, which are materials that are “capable of softening or fusing when heated and of hardening again when cooled [2],” and are commonly used in products such as this that are made through a molding process [1] [3] [4]. Rather than being destroyed or damaged by the heating, they instead “become liquid (i.e. have a “glass transition”) at a certain temperature… [and] can be heated to their melting point, cooled, and reheated again without significant degradation [4].” As a result, thermoplastics are frequently used in manufacturing processes due to how relatively easy they are to manipulate. The two thermoplastics that are used in the Rubik’s Cube are acrylonitrile butadiene styrene (ABS) and nylon [1][3]. The first, ABS, is what is classified as a terpolymer, meaning that it is comprised of three different monomers: acrylonitrile, butadiene, and styrene respectively [5].
Although they’re referred to as monomers, the three components of ABS are far more complex in regards to their creation, extraction, and manufacturing than it would initially seem. Acrylonitrile, the first of the three, is a synthetically-created compound that takes the form of a highly toxic liquid, and is frequently used in industry and manufacturing for products such as plastic [6]. While acrylonitrile is considered a raw material for ABS, it is manufactured by the Sohio process when “propene, oxygen (as air), and ammonia are catalytically converted directly into acrylonitrile using a fluidized-bed reactor [6].” Propene, also known as propylene or methyl ethylene, is a gas that is noted to smell vaguely like petroleum and is easily ignited, which can cause explosions [7]. These characteristics are appropriate however, given that propene can be obtained in several relevant ways, such as being a byproduct obtained from petroleum oils during the refining of gasoline, as a guaranteed yield of catalytic or thermal cracking of hydrocarbons, and through the catalytic degeneration of propane [7]. Ammonia is a nitrogen-based compound that is produced and procured in a multitude of ways, and used heavily in industry and manufacturing despite its associated dangers and high toxicity [8]. Ammonia has multiple methods of manufacture, such as being produced as a “byproduct of coal distillation, by the action of steam on calcium cyanamide, and from the decomposition of nitrogenous materials,” as well through a reduction process involving “atmospheric nitrogen and a hydrogen source, for example, methane, ethylene or naphtha, at high temperatures and pressures in the presence of an iron catalyst [8].” Another method of ammonia production is from the mixing of “carbon monoxide, hydrogen, carbon dioxide, and nitrogen (from air) obtained by steam reforming or by partial combustion of natural gas or from the action of steam on hot coke [8].”
The second component of ABS is butadiene, which is classified as a petroleum hydrocarbon [5]. Also known as a total petroleum hydrocarbon (TPH), this category refers to chemical compounds that originate directly from crude oil, and that are used in manufacturing processes involving petroleum and petroleum-based products [9]. Finally, the last component of ABS is styrene, which is a colorless, toxic liquid that is commonly used to manufacture products such as plastics, rubbers, and resins [10]. Styrene is created through the dehydrogenation of ethylbenzene, meaning that hydrogen is removed from the organic molecules; this results in a colorless liquid that also smells like petroleum, that in turn is mainly used for the production of styrene [5] [11]. Ethylbenzene itself is the result of the alkylation of benzene with ethylene, with benzene being found in crude oils and as a result of oil refining and ethylene being a product of steam cracking petroleum hydrocarbons [11] [12] [13].
The second kind of thermoplastic that is used in the manufacturing of the Rubik’s Cube is nylon which, although simpler than its ABS counterpart, still has more to it than meets the eye. Nylon was the first synthetic thermoplastic polymer to be commercially successful, and is still very heavily used in both the manufacturing process and in products used for daily life. As nylon is not naturally occurring in nature, this material is made entirely from carbon-based compounds found in fossil fuels such as coal and petroleum, although some attempts have been made at recreating it with renewable materials such as castor oil [14]. Although ABS and nylon are formally acknowledged as the primary plastics used in Rubik’s Cubes, there is the possibility of traces of other minor plastics that were potentially involved in the process whether intentional or not, given factory conditions that may lead to contamination and the variance of practices and settings from company to company.
After the plastic, the second-most major component of the Rubik’s Cube’s design and manufacturing is the stickers that are applied to the different faces of the cubes, indicating their color and orientation. The colors included in the traditional Rubik’s Cube are red, orange, yellow, green, blue, and white, with the primary objective of the puzzle itself being to have each color delegated to its own individual side. These stickers, arguably the most important aspect of object overall, are made of polypropylene, which is a thermoplastic synthetic resin that is created through the polymerization of propylene [13] [15]. Propylene, as previously mentioned for its role in ABS is also known as propene, and is produced as a byproduct of processes involving petroleum, hydrocarbons, and propane [7]. Polypropylene is specifically selected for this role in the object’s design due to its resilient nature, as it’s able to help prevent the degradation of the stickers’ conditions, take on the color easily and effectively, and can be produced into an easily manipulated and utilized film [3].
The third and final components that are utilized in the assembly and manufacturing of the Rubik’s Cube are the metal springs and screws that are located in the central core of the puzzle, which allow for it to both remain in one piece overall and to have the mobility required for its purpose. In following with the pattern of previous components, despite not appearing particularly complicated from exterior view, the metals that are used in these materials are often deeply complex in their makeup and production processes. The cube itself utilizes about six screws, which are usually made of low to medium carbon steel wire, but have also been known to utilize alternative, less expensive metals of equal strength, such as stainless steel, brass, and nickel or aluminum alloys [16]. Furthermore, screws can sometimes be coated with zinc, cadmium, nickel, or chromium for additional strength and protection against cracks or breaks [16]. In regards to where these materials are actually acquired from in the world, steel itself is a type of iron alloy, notable for its lower-than-usual carbon content, and around 50% of the world’s supply comes from China, where it’s mined originally as iron ore [17]. Brass is comprised of a combination of copper and zinc, with copper extracted as ore in open pit mines in locations such as Utah, New Mexico, and Chile and zinc ore being predominantly mined in Canada, Russia, Australia, the USA, and Peru [18] [19] [20]. Additionally, cadmium rarely appears on its own in nature and is usually acquired through its removal from zinc during the refining process, meaning that it originates from many of the same locations [21]. Nickel is most commonly obtained from the mining of pentlandite, and most of the world’s supply is harvested from the Sudbury region of Ontario, Canada [22]. Aluminum is the most abundant metal in the earth’s crust and is mined most commonly in Australia, Africa, South America, and the Caribbean [23]. Finally, chromium is primarily sourced from chromite, which is mined in South Africa, Kazakhstan, India, Albania, and Turkey [24].
In the manufacturing of springs, steel alloys are the most commonly used, including high-carbon, oil-tempered low-carbon, chrome silicon, and stainless steel; although other types of metal that are occasionally utilized are beryllium copper alloy and titanium [25]. The United States is responsible for mining most of the world’s beryllium, producing more than 85% of the worldwide supply in 2010 from just one mine in Utah [26]. Titanium is obtained from a variety of minerals such as rutile, ilmenite, and titanite along with many others, and is usually mined in Australia, Sierra Leone, South Africa, Russia, and Japan [27].
In conclusion, as technology has developed and become increasingly more reliant on energy and resource consumption, general awareness of manufacturing processes and the components involved has decreased to the point of anonymity. This knowledge however, in all its detail, is vital to be aware of in order to be able to fully understand the impact that the manufacturing process of a single, seemingly simple puzzle game has on the environment and its resources and how interconnected it is to the rest of the industrial complex. Despite first impressions dictating that the Rubik’s Cube is made only of simple plastic and some metal parts, it’s only when all the facts are known that a more profound perspective can be achieved regarding how manufacturing and industry has shaped society, values, the environment, and impacted the earth and its resources.
Works Cited:
1. “Rubik's Cube.” How Products Are Made, www.madehow.com/Volume-7/Rubik-s-Cube.html.
2. “Thermoplastic.” Merriam-Webster, Merriam-Webster, www.merriam-webster.com/dictionary/thermoplastic.
3. “Rubik's Cube: How It's Made and Solution Clues - Craftech Industries - High-Performance Plastics - (518) 828-5001.” Craftech Industries, 9 July 2018, www.craftechind.com/rubiks-cube-made-solution-clues/.
4. Rogers, Tony. “Creative Mechanisms Blog .” Everything You Need to Know About ABS Plastic, www.creativemechanisms.com/blog/everything-you-need-to-know-about-abs-plastic.
5. “ABS: Acrylonitrile Butadiene Styrene - Craftech Industries - High-Performance Plastics - (518) 828-5001.” Craftech Industries, 30 May 2018, www.craftechind.com/abs-acrylonitrile-butadiene-styrene/.
6. “Acrylonitrile.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/acrylonitrile.\
7. “Propylene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/Propene.
8. “Ammonia.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/ammonia.
9. “Toxic Substances Portal.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 3 Mar. 2011, www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=75.
10. “Styrene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/styrene#section=Top.
11. “Ethylbenzene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/ethylbenzene.
12. “Benzene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/benzene#section=Methods-of-Manufacturing.
13. “Ethylene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, pubchem.ncbi.nlm.nih.gov/compound/ethylene#section=Methods-of-Manufacturing.
14. “Nylon - The Science of Synthetic Textiles.” Explain That Stuff, 7 Feb. 2018, www.explainthatstuff.com/nylon.html.
15. Staff, Creative Mechanisms. “Creative Mechanisms Blog .” Everything You Need To Know About CNC Machines, www.creativemechanisms.com/blog/all-about-polypropylene-pp-plastic.
16. "Screw.". “Screw.” How Products Are Made, Encyclopedia.com, 2018, www.encyclopedia.com/science-and-technology/technology/technology-terms-and-concepts/screw.
17. “Iron and Steel - Introduction to Their Science, Properties, Uses.” Explain That Stuff, 12 Aug. 2018, www.explainthatstuff.com/ironsteel.html.
18. “Brass.” How Products Are Made, www.madehow.com/Volume-6/Brass.html.
19. Hamilton, Jason. “Copper.” ScienceViews.com, scienceviews.com/geology/copper.html.
20. “Zinc.” Lenntech Water Treatment & Purification, www.lenntech.com/periodic/elements/zn.htm.
21. “Cadmium.” Minerals Education Coalition, mineralseducationcoalition.org/minerals-database/cadmium/.
22. “The Element Nickel.” Jefferson Lab, education.jlab.org/itselemental/ele028.html.
23. Harris, William. “How Aluminum Works.” HowStuffWorks Science, HowStuffWorks, 8 Mar. 2018, science.howstuffworks.com/aluminum2.htm.
24. “Chromium.” Minerals Education Coalition, mineralseducationcoalition.org/elements/chromium/.
25. “Springs.” How Products Are Made, www.madehow.com/Volume-6/Springs.html.
26. Blaszczak-Boxe, Agata. “Facts About Beryllium.” LiveScience, Purch, 6 Oct. 2017, www.livescience.com/28641-beryllium.html.
27. “Titanium.” Minerals Education Coalition, mineralseducationcoalition.org/elements/titanium/.
Leo McGrath
DES 40A
Professor Cogdell
3 December 2018
Rubik’s Cube: Embodied Energy
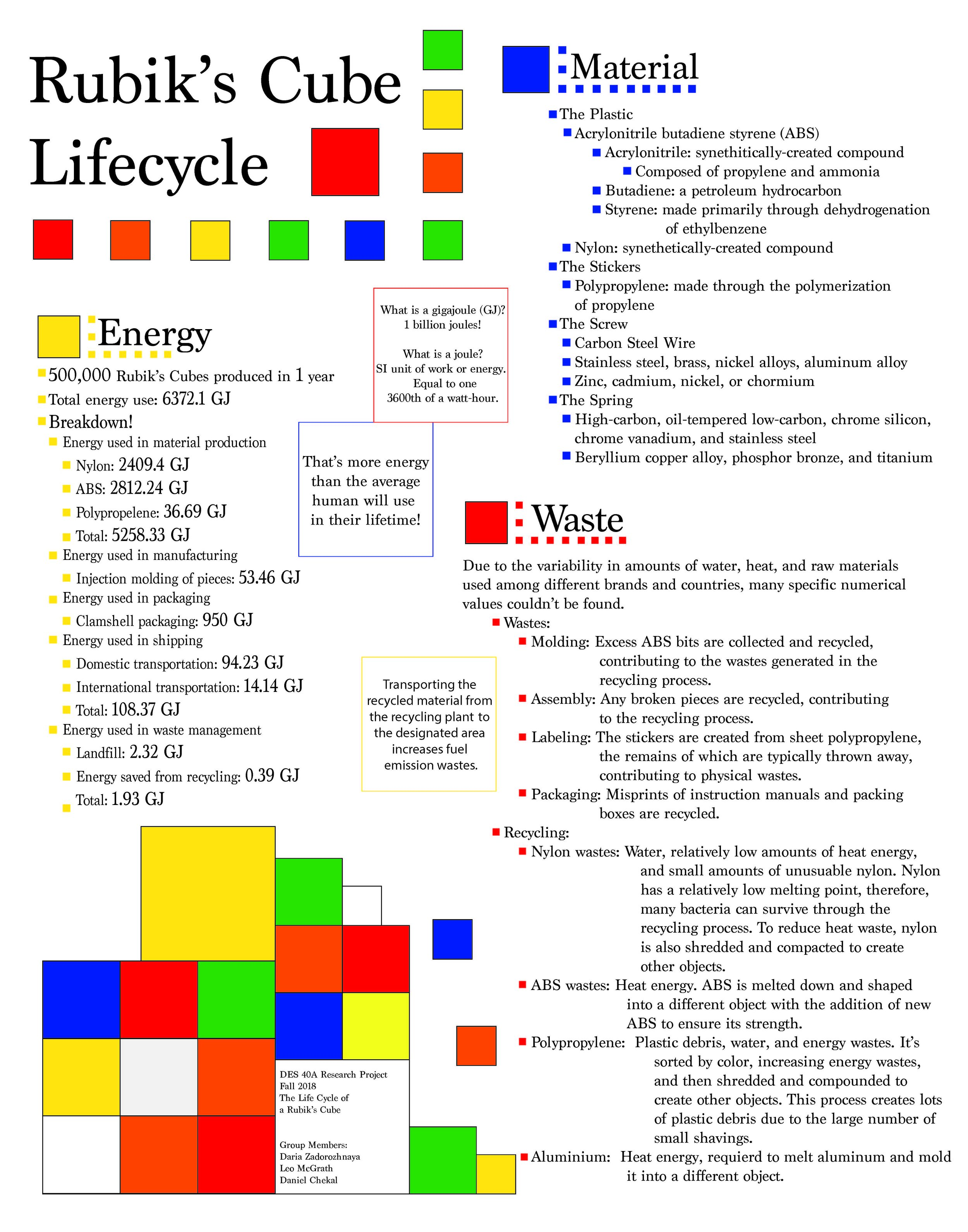
A Rubik’s cube is a popular puzzle toy that, although simple in appearance, easily stumps anyone who attempts to solve one without knowing the proper steps. The Rubik’s cube was designed by Erno Rubik in the 1970s and grew very popular in the 1980s selling 200 million cubes over a 3-year time frame between 1980 and 1983. To this day over 500,000 cubes are sold worldwide each year (“Rubik’s Cube”, 2018). As the name implies, a Rubik’s cube is a cube-shaped toy with 6 different colored faces. Each face of the cube is broken into 9 squares each of which is colored. The faces can be turned in either direction allowing for the complete scrambling of the Rubik’s cube with the goal being to unscramble the cube back to its original state. Although the solving of a Rubik’s cube may be fairly complex, the design is more simple. The 6 centerpieces of each of the faces of a Rubik’s cube are fixed to the core of the cube and can rotate. The remaining 20 side pieces aren't fixed to anything and instead are held in place by tabs which connect to circular tracks built into the other pieces (“Rubik’s Cube”, 2018). This ingenious design is simple enough that a popular method for solving a Rubik's cube without having to know how to is simply by taking the pieces apart and putting them back together again, solved. The design of a Rubik’s cube may make it seem like a simple cube of plastic, however, by going over the life cycle of a Rubik’s cube and studying the energy use of each step along the way, this paper will show that the embodied energy of a Rubik’s cube is more than its simple nature suggests.
The production of raw materials is a very energy intensive step in the life cycle of a Rubik’s cube due to it being made primarily out of plastic. According to makehow.com, the primary plastics used in a Rubik's cube are Nylon, Acrylonitrile butadiene styrene (ABS), and Polypropylene. Nylon, being the densest of the three plastics, is used for the core of a Rubik's cube to which all the other pieces attach and rotate. ABS is used for the side pieces which make up most of the cube. ABS is lighter than Nylon but still has the strength to make up structural parts of the cube. Finally, Polypropylene is used for the colored stickers that are placed into each face of the side pieces (“Rubik’s Cube”, 2018). To estimate the embodied energy of a Rubik's cube this paper will assume a Rubik's cube weighs 100 grams. This number was calculated by averaging the displayed weight of several product listings for Rubik's cubes on the popular online marketplace amazon.com. This placed the weight between 170 and 200 grams. Assuming 40-50% of that weight was due to packaging, documentation, and the included stand gives the estimate of 100 grams for the weight of a Rubik's cube. The total weight of the cube is then split off into the core, the side pieces, and the stickers. The core is assumed to be approximately 40% of the weight due to the density of Nylon and the core being a solid part. 59% of the weight is attributed to the side pieces. This number may seem low compared to the core for how many side pieces there are, however, this is due to ABS being a less dense plastic and that the side pieces are more hollow than the core. The final 1% of the weight is the Polypropylene stickers. It should be noted that a Rubik's cube contains several metal screw and springs in its core but these will be overlooked due to limited information on the screws and their production. The final piece of the foundation to be laid out is that all the calculations and energy estimates in this paper will be done on the baseline amount of Rubik’s cubes produced in a year, which is 500,000. With these guidelines in mind, estimates can be made about the energy use of plastic production.
Of the three plastics in a Rubik’s cube, Nylon uses the most energy to produce. 20,000 kg of Nylon is used in the production of Rubik’s cubes each year. According to a report for PlasticsEurope (2005a), the production of 1 kg of Nylon uses 120.47 MJ of energy. The three largest sources of which are coal at 12.28 MJ, oil at 38.57 MJ and gas at 70.13 MJ (Boustead,2005a). It should be noted that 11.19 MJ of energy is recovered which is why the total of coal, oil, and gas alone account for a little over 100% of the energy used before factoring in this recovered energy. The production of 20,000 kg of Nylon uses 2409.4 GJ with over 90% of that energy coming from fossil fuels. Next is the 29500 kg of ABS used in the side pieces of Rubik’s cubes. A PlasticsEurope report done on ABS (2005b) puts the energy use for the production of 1 kg of ABS at 95.33 MJ. The two major energy sources being oil at 44.10 MJ, and gas at 45.30 MJ. Interestingly, only 5.32 MJ of energy comes from coal which is comparable to the amount of energy derived from nuclear fuels in the production of Nylon. Additionally, only 3.98 MJ of energy is recovered in the production of ABS (Boustead, 2005b). In total, the production of 29500 kg of ABS uses 2812.24 GJ of energy, once again with over 90% of the energy being derived from fossil fuels. Finally, the 27 million individual stickers that give color to the faces of Rubik’s cubes use 500 kg of Polypropylene. PlasticsEurope (2005c) reports that 1 kg of Polypropylene takes 73.37 MJ of energy to produce. Oil and gas are again the two largest sources of energy with 45.85 MJ and 23.83 MJ of energy coming from each respectively. Additionally, 1.91 MJ of energy is recovered throughout the production of Polypropylene (Boustead, 2005c). 36.69 GJ of energy is used in the production of 500 kg of Polypropylene. Totally, the plastics used in 500,000 Rubik’s cubes take 5258.33 GJ of energy to produce almost all of which comes from fossil fuels. The production of plastic uses by far the highest amount of energy in the life cycle of a Rubik’s cube, especially when compared to manufacturing.
Although overall Rubik’s cubes have a relatively high embodied energy, the manufacturing process uses relatively little energy due to the ease at which plastics can be molded. The primary method for the manufacturing of the pieces of a Rubik’s cube is through injection molding (“Rubik’s Cube”, 2018). Injection molding is the process of injecting molten plastic into a mold. It allows for large-scale manufacturing and produces less waste than traditional manufacturing methods like CNC manufacturing (Rogers). The company PlastikCity provides a useful tool which estimates the energy usage of injection molding (“Injection Moulding”, 2018). The tool takes the inputs of weight, cycle time, cost of energy and total hours run to give an estimated cost. The cycle time for the manufacturing of Rubik’s cube parts is 20 seconds (“Rubik’s Cube”, 2018). To get the energy usage rather than the price, the cost per kWh is set to 1. The individual weight and hours ran can vary as long as 49,500 kg of parts are produced each year. For a weight of 35 grams per cycle, 7860 hours of production are required. This is the equivalent of running an injection molding machine nonstop for 90% of the year. With these parameters, the manufacturing of Rubik’s cube parts takes 53.46 GJ when manufactured with efficient modern machines that run entirely off of electricity (“Injection Moulding”, 2018). Worldwide the main sources of energy for electricity production comes from fossil fuels at 66% of energy production. Hydroelectric and nuclear are the second highest sources of electricity production at 17% and 11% of production respectively (“Breakdown of Electricity Generation”). Once the pieces of a Rubik’s cube are manufactured they will be fitted together into the completed cubes. The method used to do so is unclear, the pieces could be fitted together by human hands or by a machine. Similarly, the application of the Polypropylene stickers is not discussed much. This is likely done by a machine due to the level of precision needed to achieve a uniform product. Due to the lack of information on the piecing together of the Rubik’s cubes and the application of stickers, energy contributions from these steps will be overlooked. Even with the likely significant increase in energy use from the assembly, manufacturing is still a very small contributor to the large embodied energy of a Rubik’s cube. The low energy use of manufacturing is an outlier in the life cycle of a Rubik’s cube, with the next step, packaging, showing how excessive modern consumerism can be.
Common packaging used for Rubik’s cubes is wastefully energy intensive. The most common packaging used is blister clamshell packaging (“Rubik’s Cube”, 2018). This type of packing encases the Rubik’s cubes in a thin layer of hard plastic. The plastic used can vary but Polypropylene, Polyethylene terephthalate (PET), and Polylactic acid (PLA) are all popular plastics to use. A study on plastic clamshell packaging puts the energy usage for the production of 1000 clamshells at 1802 MJ for PLA, 1846 MJ for Polypropylene and 2040 MJ for PET (“Summary of Life Cycle Assessments” 11). Assuming about 1900 MJ of energy is used for the production of 1000 clamshells, packaging of all 500,000 Rubik’s cubes will use 950 GJ of energy. It should be noted that this estimate is based on the figures given for if all the clamshells were landfilled rather then recycled. Recycling the clamshells could lead to a net energy usage up to 50% lower than this estimate. However, either way, the energy used in packaging is still very large compared to how short a length of time the packaging is used for on commercial products like Rubik’s cubes. There are alternative options for packaging when purchasing a Rubik’s cubes which are presumably more energy efficient and eco-friendly. These alternatives will be overlooked since there is little information on this packaging with the official Rubik’s cube web store describing the packaging simply as “Green”. Furthermore, these alternative forms of packaging aren’t as prevalent as clamshell packaging on consumer products. Clamshell packaging is seen on all sorts of different consumer goods, significantly increasing the embodied energy of each product it is used on. Despite how wasteful packaging can be, it is still necessary for protecting the Rubik’s cubes as they are shipped from the factory to store shelves.
Due to the worldwide market of Rubik’s cubes, the shipping from factory to store shelves is another large energy user. It is not clear where exactly Rubik’s cubes are produced. However, it can safely be assumed that Asian countries with high levels of manufacturing such as China and Vietnam are most likely where both the materials and the Rubik’s cubes are produced. Due to lack of information, the specifics of transportation between the production of materials and the factories will be left out. With this in mind, energy estimates will be made for both maritime international freight and domestic freight. Data provided by the United States Bureau of Transportation Services gives the total energy usage of the U.S. transportation sector in the year 2015 to be 27.38 quadrillion British thermal units (quads) (“U.S. Energy Consumption”). In the same year, the Bureau reports that 16,896 millions of tons of goods were transported by the U.S transportation sector (“General”). Changing units from quads to MJ and from tons to kilograms gives an estimated 1.88 MJ of energy per kg of freight. This gives an estimate of 94.23 GJ of energy used to transport 50,000 kg of Rubik’s cubes domestically. Additionally, to get to far away markets Rubik’s cubes will need to be shipped by sea. According to the International Energy Agency, maritime shipping uses “on average about 15% as much energy as heavy-duty trucks” (“Average Freight Energy Intensity”). Adding in an additional 15% of the energy used gives an estimate of 108.37 GJ of energy used for transportation overall. This estimate leaves out a lot. It doesn’t account for the distances that the Rubik’s cubes may be shipped; it doesn’t account for the different types of land transportation such as trucking and rail; it also doesn’t factor in the transportation of the raw plastics to the factories. Since so much is left out, this estimate should be taken as a very rough baseline. As with most of the energy used so far, the largest sources of energy for transportation are fossil fuels at 92%. The transportation sector uses a high amount of petroleum products with 89% of the energy coming from them. Only 3% of the energy comes from gas and coal isn’t even on the chart (“Energy Use for Transportation.”). All this burning of fossil fuels achieves the effect of putting the Rubik’s cubes in the hands of consumers, where the toys will be played with until it is time for them to be thrown away. At that point, a Rubik’s cube reaches the final step in its life cycle, waste management.
Plastics are built to last hundreds of years and can be reused, this makes waste management a potential area for Rubik’s cubes to reduce their large embodied energy. There is a constant demand for plastics of all types and the makeup of plastics means that they can be recycled and reused. However, despite the large potential that the recycling of plastics has to reduce energy use, only about 9% of plastics are recycled worldwide each year (Nairobi, 2018). Assuming a similar percentage of Rubik’s cubes were recycled, 45,500 kg of Rubik’s cubes would go to landfills with only 4,500 kg being recycled. According to a life cycle analysis of landfill systems, 45,500 kg of waste would use about 2.32 GJ of energy with over half of this energy use coming from transportation to landfills (Nabavi-Pelesaraei, Ashkan et al.). This estimate doesn’t take into account energy recovery from the combustion of waste which, according to this life cycle analysis, can recover roughly 8% of the energy used in landfill systems. It is unclear how much waste is combusted worldwide so this potential energy saving will be left out. An EPA fact sheet estimates that 50-75 Million British thermal units of energy is saved per ton of plastic recycled (“Fact Sheet Recycling the Hard Stuff.”). This gives the recycled 4,500 kg of Rubik’s cube waste an energy saving of .392 GJ. This number could easily be brought higher and with proper waste management and recycling up to 4.36 GJ of energy could be saved from the recycling of Rubik’s cube waste.
Overall, the total amount of energy used in the life cycle of one year’s worth of Rubik’s cubes (500,000) comes out to 6372.1 GJ. To put this number into context in the year 2015 humans on average used 73 million Btu or 77.08 GJ of energy per person (Wirfs-Brock). The World Health Organization reports that the world life expectancy is 72 years (“Life Expectancy.”). The average person, therefore, will use 5549.76 GJ of energy in their lifetime. The life cycle of just one year’s worth of Rubik’s cubes uses more energy than an average person does in their entire life. As this paper has shown, the embodied energy of a Rubik’s cube is higher than one would think. The level of energy use in the modern world plays a part in this with plastic products being seen as cheap alternatives despite their large energy usage. Looking at the life cycle of a seemingly simple product shows that consumer products have a lot of room to decrease their embodied energy. This is especially true for plastic production. As shown, the production of plastics is extremely energy intensive with its energy use making up over 80% of the total energy use of Rubik’s cubes. Better methods of production and reuse of plastics that can drastically bring down this number could potentially have a major positive impact on the world’s energy use.
Bibliography
“A Summary of Life Cycle Assessments (LCAs) and Life Cycle Inventories (LCIs)” Losgatos.granicus.com. City of San Jose, 2013. Web. 28 Oct. 2018,http://losgatos.granicus.com/MetaViewer.php?view_id=5&clip_id=1321&meta_id=137201.
“Average Freight Energy Intensity and Activity.” IEA, International Energy Agency, 3 July 2017, www.iea.org/newsroom/energysnapshots/average-freight-energy-intensity-and-activity.html.
Boustead, I. (2018). “Eco-profiles of the European Plastics Industry Acrylonitrile-Butadiene-Styrene Copolymer (ABS) .” Inference.org.uk. PlasticsEurope, 2005a. Web 28 Oct. 2018, http://www.inference.org.uk/sustainable/LCA/elcd/external_docs/abs_311147f0-fabd-11da-974d-0800200c9a66.pdf.
Boustead, I. (2018). “Eco-profiles of the European Plastics Industry POLYAMIDE 6 (Nylon 6) .” Inference.org.uk. PlasticsEurope, 2005b. Web 28 Oct. 2018a, http://www.inference.org.uk/sustainable/LCA/elcd/external_docs/n6_311147f6-fabd-11da-974d-0800200c9a66.pdf.
Boustead, I. (2018). “Eco-profiles of the European Plastics Industry POLYPROPYLENE (PP).” Inference.org.uk. PlasticsEurope, 2005c. Web 28 Oct. 2018b, http://www.inference.org.uk/sustainable/LCA/elcd/external_docs/pp_31116f04-fabd-11da-974d-0800200c9a66.pdf.
“Breakdown of Electricity Generation by Energy Source.” Breakdown of Electricity Generation by Energy Source | The Shift Project Data Portal, Web. 28 Oct. 2018, www.tsp-data-portal.org/Breakdown-of-Electricity-Generation-by-Energy-Source#tspQvChart.
“Energy Use for Transportation.” Factors Affecting Gasoline Prices - Energy Explained, Your Guide To Understanding Energy - Energy Information Administration, U.S. Department of Energy, 23 May 2018, www.eia.gov/energyexplained/?page=us_energy_transportation.
“Fact Sheet Recycling the Hard Stuff.” Archive.epa.gov. EPA, 2002. Web. 28 Oct. 2018, https://archive.epa.gov/epawaste/nonhaz/municipal/web/pdf/f02023.pdf.
“General.” Number of U.S. Aircraft, Vehicles, Vessels, and Other Conveyances | Bureau of Transportation Statistics, Bureau of Transportation Statistics, www.bts.gov/bts-publications/freight-facts-and-figures/freight-facts-figures-2017-chapter-2-freight-moved.
“Injection Moulding Machine Energy Consumption Calculator.” Material Melt and Mould Temperature Chart | PlastikCity, PlastikCity Ltd, 2018, Web. 25 Nov. 2018, www.plastikcity.co.uk/useful-stuff/energy-consumption-calculator.
“Life Expectancy.” World Health Organization, World Health Organization, 6 July 2018, www.who.int/gho/mortality_burden_disease/life_tables/situation_trends/en/.
“Plastics: Material-Specific Data.” EPA, Environmental Protection Agency, 19 July 2018, www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data.
Rogers, Tony. “Creative Mechanisms Blog .” Everything You Need To Know About CNC Machines, www.creativemechanisms.com/blog/everything-you-need-to-know-about-injection-molding.
“Rubik's Cube.” How Products Are Made, Advameg, Inc., 28 Oct. 2018, www.madehow.com/Volume-7/Rubik-s-Cube.html.
“U.S. Energy Consumption by the Transportation Sector.” Number of U.S. Aircraft, Vehicles, Vessels, and Other Conveyances | Bureau of Transportation Statistics, BTS, 2017, www.bts.gov/content/us-energy-consumption-transportation-sector.
Nabavi-Pelesaraei, Ashkan et al. "Modeling Of Energy Consumption And Environmental Life Cycle Assessment For Incineration And Landfill Systems Of Municipal Solid Waste Management - A Case Study In Tehran Metropolis Of Iran." Journal of Cleaner Production 148 (2017): 427-440. Web. 30 Oct. 2018, https://www.sciencedirect.com/science/article/pii/S0959652617301944.
Nairobi. “UN Warns Globally Only 9 Percent of Plastic Waste Is Recycled.” Www.efe.com, Agencia EFE, 5 June 2018, www.efe.com/efe/english/portada/un-warns-globally-only-9-percent-of-plastic-waste-is-recycled/50000260-3638548.
Wirfs-Brock, Jordan. “Energy Explained: Where Does It Come From And How Much Do We Use?” Inside Energy, 19 Dec. 2017, insideenergy.org/2017/01/12/energy-explained/.
Daniel Chekal
Professor Cogdell
SAS 43
6 December 2018
Life Cycle Paper Outline - Wastes
From almost any toy store or supermarket across the world, people can get many different shapes and sizes of Rubik’s Cubes. The complexity and the seemingly unending number of moves available creates a unique type of puzzle that can be enjoyed by any generation. However, there are many small parts that go into the molding of the structure and creation of the colored stickers on the toy. Furthermore, once the owner is either too frustrated to try any more or found the secret to the toy, it is often recycled and this, contrary to common belief, creates even more additional waste. Unfortunately, due to the variability in amounts of water, heat, and raw materials used for different brands, countries, sizes, and shapes of a Rubik’s Cube, many specific numerical values couldn’t be found. The Rubik’s Cube, a beloved or despised puzzle, surprisingly has a surprising amount of wastes that aren’t very apparent, of which include material wastes, packing wastes, and recycling wastes.
Nylon
Being one most used products in the production of the Rubik’s cube, it’s fitting that I start with nylon 6-6, which proves to have more wastes than what’s typically thought of when seen at a glance. It is a synthetic fiber that is created by the combination of multiple monomers in a chain, creating a polymer that can be up to 20,000 units long [1]. The chemical compounds that go into the production of nylon are hexamethylenediamine and adipic acid, which combine to create nylon’s chemical formula and water as a by-product. Both hexamethylenediamine and adipic acid are regarded as acutely toxic to humans, the former being more potent and having more effects. For example, workers who come into contact with hexamethylenediamine can expect significant amounts of skin and eye irritation while continued exposure may cause respiratory problems [2]. Adipic acid is less toxic and without many effects. Although there aren’t many effects on skin contact, there are some irritation when it comes into contact with eyes and may accumulate if inhaled and irritate both the lungs and nose [3]. The chemical formula of the production of nylon is C6H16N2 + C6H10O4 = (C12H22N2O2)N + 2(H2O) and shows how for each molecule of nylon created, two molecules of water are created [4]. This by-product of water creates the need for nylon production plants to treat and dispose of this water effectively since it comes into contact with both chemicals. Although there are drawbacks in the handling of the products, the strength of nylon makes up for it since it is not only durable but also resistant to many fluids, such as oils, alcohols, and water [5].
Acrylonitrile Butadiene Styrene (ABS)
Being another major component in the plastic that’s used to make Rubik’s Cubes, Acrylonitrile Butadiene Styrene (ABS) has significant amounts of wastes since it’s a plastic composed of three different types of monomers: acrylonitrile, butadiene, and styrene. Different amounts of these three monomers create different types of plastic and each contribute different beneficial properties to the product. Acrylonitrile gives the product both chemical and thermal strength while butadiene provides more strength and styrene gives the plastic its glossy aspect [6].
Acrylonitrile is a man-made chemical that has the structure of C3H3N and is typically found in the air near chemical plants. This chemical typically evaporates very fast and therefore don’t have much of an impact on human health. If small amounts are inhaled by humans, mild irritation may occur and may cause peeling or blistering if it comes in contact with human skin. However, in rare cases, where large amounts are consumed, deaths have been reported; unfortunately, there isn’t a specific amount of intake that causes death and affects people differently so everyone needs to be wary of contact with this chemical. Furthermore, in cases of extreme amounts of exposure, symptoms may include temporary damage to red blood cells and the liver while also causing headaches; these symptoms fortunately disappear once the person is no longer in contact with the chemical [7].
Butadiene is also a synthetic gas that has a chemical structure of C4H6 and found in urban and suburban areas. This chemical is also produced by motor vehicle exhaust but can be very hazardous for those in contact. For example, prolonged exposure can cause symptoms including irritation in the eyes, nose, throat, coughing, lightheadedness, and reproductive effects. Even more prolonged exposure to the gaseous version of butadiene can cause cancer and both respiratory and heart diseases. Furthermore, the liquid version of butadiene can cause frostbite if it comes into contact with human skin [8].
The last chemical in ABS is styrene, an organic compound with the chemical formula of C8H8. This chemical is also very toxic to humans who work or come into contact with it and can also be found in reinforced plastics and polystyrene factories. With small amounts of exposure, humans can expect eye irritation, gastrointestinal effects, and oral irritation. For patients who are exposed for longer periods of time, internal organs such as the liver, kidney, and stomach experience irritation. Exposure can also damage red blood cells and reproductive cells. Furthermore, cancers can also arise from exposure, including, but not limited to lung cancer, leukemia, lymphoma, and is considered to be a human carcinogen [9].
As evidenced, the toxic side effects of dealing with the individual components of ABS, one of the most used components in the Rubik’s Cube, can be staggering. However, they become less apparent and impactful to humans once they are combined into ABS because it requires temperatures above 200 degrees Celcius for ABS to create these toxic fumes that affect humans [10]. Further increases in toxicity once it is combined with other components, such as nylon and makes the beloved toy safe for users.
Polypropylene
The last major component of a Rubik’s Cube is a polymer plastic called polypropylene (PP) and has very vague yet prominent toxic wastes associated with its production. It has the chemical formula of (C3H6)N and are popular due to their versatility, flexibility, and high heat resistance. This enables PP to be very versatile and used in a wide range of products [11]. During the process of creating PP, a lot of emissions, such as sulfur oxides, methanol and nitrous oxides are released into the atmosphere, polluting the environment around the production plant. Additionally, many other by-products, such as plastic resin, can have even more detrimental impacts on the environment by permanently hardening around something. Even disregarding the by-products that are detrimental to the environment, the production of PP can have lasting negative impacts on the health of workers. The range of effects on humans are very wide due to PP’s ability to mix with other additives and change the chemical composition of itself, which can have a new set of harmful effects on workers.[12].
Manufacturing, Shipping, and Packaging
Creating the simple toy requires the following steps of molding, assembly, labeling, and packaging, all of which produce all produce different types of wastes.
Molding
The plastic pieces made of acrylonitrile butadiene styrene (ABS) is melted and molded into the pieces required [13]. The remaining plastic bits are collected and melted again to make more Rubik’s cube parts. These plastic parts are typically made of recycled plastics. This generates a lot heat wastes due to the lack of an efficient heating system as well as the toxic fumes generated when ABS pieces are melted down.
Assembly
The core parts are glued together and then the other pieces are assembled around the core. Any broken pieces will be put back into the molding part and melted down to be recreated. Although not specifically mentioned in the previous section of the report, glue is used to secure the plastic cover and general framing of the cube. This process doesn’t produce a lot of wastes due to the uniformity and computerization of the process at this point.
Labeling
The stickers are created from sheet polypropylene (PP) which is then colored and laminated. It is then stuck onto each piece of the cube. These polypropylene sheets are cut and the remains are typically thrown away, increasing the wastes. This is detrimental because the creation of PP creates a lot of toxic wastes that negatively affect not only the environment but also the workers creating PP. The lack of utilizing every available piece of PP increases wastes significantly since the increase in Rubik’s Cube production continuously increases the amount of PP wasted.
Packaging
The cube is finally put into a cardboard or paper box and an instruction manual is frequently added as well. These pieces of paper all require prints that all require ink. Potential misprints not only waste large amounts of cardboard, paper, and ink, but are also then introduced to the recycling process, which generates even more wastes that could’ve been avoided had the initial print been accurate. [14]
Throughout this whole process, the quality of the toy is monitored and these checks help weed out production errors. However, the large number of checks consequently creates the need to use more energy to ensure the product is satisfactory [15].
Recycling
The recycling process has multiple steps that each produce wastes on the way. The process involves sorting, washing, shredding, identification & classification, and extruding and this requires a lot of heat and water. Unfortunately, due to the nature of nylon, a lot of water is wasted in its recycling process. Since it has a relatively low melting point of about 400 degrees Fahrenheit, a lot of bacteria on the nylon can survive the melting process and get transferred to the next material it becomes. Therefore, nylon must be cleaned thoroughly before its melted and remade into something else. This creates a lot of water wastes that must be disposed of effectively and responsibly, further increasing the amount of energy wasted [16]. To recycle Acrylonitrile Butadiene Styrene (ABS), it is melted down, wasting heat, and shaped into a different object with the addition of new ABS to ensure its strength. The addition of new ABS creates more wastes because a portion of the ABS generated in its production process is used to strengthen the recycled ABS, rather than used in materials, requiring more ABS to be made to make up for the loss of supply [17]. To recycle polypropylene, needs to be separated through a “sink-float” test where its unique density enables it float and be removed from the rubbage easily. Polypropylene is sorted by color and then shredded and compounded to create other objects. This process creates a lot of plastic wastes due to the large amounts of small shavings [18]. A lot of heat is required to melt aluminum and molded into a different object. Furthermore, transporting the material from the recycling plant to the designated area contributes to global warming because most of the transportation is through ground transportation. [19]
The Rubik’s Cube is a puzzle made for all ages, surprisingly has many physical wastes, not only from combining the materials to create a cube but to also create those materials from chemicals, as well as manufacturing, packaging, and recycling wastes. The complexity of the parts required to create this seemingly simple toy as well as the amount of questionable components used in the production of the toy was very shocking. This process has developed a sense of awe regarding the lack of knowledge we as consumers have regarding the amount of physical and environmental harm produced as well as the amount of wastes generated in order to create such a seemingly simple toy.
Sources Cited:
1. “How Is Nylon Made?” OpenLearn, The Open University, 26 Sept. 2005,
www.open.edu/openlearn/science-maths-technology/science/chemistry/how-nylon-made.
2. GL, Kennedy. “Toxicity of Hexamethylenediamine.” Current Neurology and
Neuroscience Reports., U.S. National Library of Medicine, 2005, www.ncbi.nlm.nih.gov/pubmed/15720033.
3. GL, Kennedy. “Toxicity of Adipic Acid.” Current Neurology and Neuroscience Reports.,
U.S. National Library of Medicine, May 2002, www.ncbi.nlm.nih.gov/pubmed/12024802.
4. “Nylon.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 3 Mar. 2017,
www.britannica.com/science/nylon.
5. Barker, Lesley. “Where Does Nylon Come From?” Sciencing.com, Sciencing, 13 Mar.
2018, sciencing.com/does-nylon-come-4596037.html.
6. Flynt, Joseph. “What Is ABS? Acrylonitrile Butadiene Styrene Properties & Uses.” 3D
Insider, 10 Nov. 2017, 3dinsider.com/what-is-abs/.
7. “Toxic Substances Portal - Acrylonitrile.” Centers for Disease Control and Prevention,
Centers for Disease Control and Prevention, 21 Jan. 2015, www.atsdr.cdc.gov/phs/phs.asp?id=445&tid=78.
8. “1,3-Butadiene.” National Center for Biotechnology Information. PubChem Compound
Database, U.S. National Library of Medicine, 2005, pubchem.ncbi.nlm.nih.gov/compound/1_3-butadiene#section=Top.
9. “Styrene.” Environmental Protection Agency,
www.epa.gov/sites/production/files/2016-09/documents/styrene.pdf.
10. “Toxicity of ABS Plastic in 3D Printing.” Whiteclouds 3D Printing, 2013,
ss.whiteclouds.com/blog/toxicity-abs-plastic-3d-printing.
11. “Process Analytics in Polypropylene (PP) Plants.” Industry USA Siemens, 2007,
www.industry.usa.siemens.com/automation/us/en/process-instrumentation-and-analytics/process-analytics/pa-case-studies/documents/cs_process_analytics_in_pp_plants.pdf.
12. “PTF: ENVIRONMENTAL IMPACTS.” Ecology Center,
ecologycenter.org/plastics/ptf/report3/.
13. “Rubik's Cube: How It's Made and Solution Clues.” Craftech Industries, 30 Oct. 2018,
www.craftechind.com/rubiks-cube-made-solution-clues/.
14. “Rubik's Cube.” How Products Are Made,
www.madehow.com/Volume-7/Rubik-s-Cube.html.
15. Chandrasekaran, Shub. “Manufacturing.” The Rubik's Cube, 12 Feb. 2012,
shubchandrasekaran.wordpress.com/2012/02/12/manufacturing/.
16. Shearlock, Carolyn. “Silicone or Nylon Cooking Tools?” The Boat Galley, 14 Dec. 2012,
theboatgalley.com/silicone-or-nylon-cooking-tools/.
17. Flynt, Joseph. “What Is ABS? Acrylonitrile Butadiene Styrene Properties & Uses.” 3D
Insider, 10 Nov. 2017, 3dinsider.com/what-is-abs/.
18. Hopewell, Jefferson, et al. “Plastics Recycling: Challenges and Opportunities.” NCBI, 27
July 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2873020/.
19. Zafar, Salman. “Methods for Aluminium Recycling.” EcoMENA, 22 Feb. 2017,
www.ecomena.org/recycling-aluminium/.
Bibliography:
1. “1,3-Butadiene.” National Center for Biotechnology Information. PubChem Compound
Database, U.S. National Library of Medicine, 2005, pubchem.ncbi.nlm.nih.gov/compound/1_3-butadiene#section=Top.
2. Barker, Lesley. “Where Does Nylon Come From?” Sciencing.com, Sciencing, 13 Mar.
2018, sciencing.com/does-nylon-come-4596037.html.
3. Boyle, Lisa Kaas. “Recycling Plastic: What a Waste.” The Huffington Post,
TheHuffingtonPost.com, 25 May 2011,
www.huffingtonpost.com/lisa-kaas-boyle/recycling-plastic-what-a_b_287673.html.
4. Chandrasekaran, Shub. “Manufacturing.” The Rubik's Cube, 12 Feb. 2012,
shubchandrasekaran.wordpress.com/2012/02/12/manufacturing/.
5. Chhabra, Esha. “Recycling Nylon Is Good for the Planet – so Why Don't More
Companies Do It?” The Guardian, Guardian News and Media, 18 May 2016, www.theguardian.com/sustainable-business/2016/may/18/recycling-nylon-bureo-patagonia-sustainable-clothing.
6. Croke, Bridget. “Developing a Polypropylene Recycling Infrastructure.” Waste360, 8
June 2017, www.waste360.com/plastics/developing-polypropylene-recycling-infrastructure.
7. Flynt, Joseph. “What Is ABS? Acrylonitrile Butadiene Styrene Properties & Uses.” 3D
Insider, 10 Nov. 2017, 3dinsider.com/what-is-abs/.
8. GL, Kennedy. “Toxicity of Adipic Acid.” Current Neurology and Neuroscience Reports.,
U.S. National Library of Medicine, May 2002, www.ncbi.nlm.nih.gov/pubmed/12024802.
9. GL, Kennedy. “Toxicity of Hexamethylenediamine.” Current Neurology and
Neuroscience Reports., U.S. National Library of Medicine, 2005, www.ncbi.nlm.nih.gov/pubmed/15720033.
10. Hopewell, Jefferson, et al. “Plastics Recycling: Challenges and Opportunities.”
Philosophical Transactions of the Royal Society B: Biological Sciences, The Royal Society, 27 July 2009, www.ncbi.nlm.nih.gov/pmc/articles/PMC2873020/.
11. “How Is Nylon Made?” OpenLearn, The Open University, 26 Sept. 2005,
www.open.edu/openlearn/science-maths-technology/science/chemistry/how-nylon-made.
12. LeBlanc, Rick. “Get an Introduction to Metal Recycling.” The Balance Small Business,
The Balance Small Business, 3 Dec. 2018, www.thebalancesmb.com/an-introduction-to-metal-recycling-4057469.
13. LeBlanc, Rick. “Polypropylene Recycling - An Introduction.” The Balance Small
Business, The Balance Small Business, 16 Sept. 2018, www.thebalancesmb.com/an-overview-of-polypropylene-recycling-2877863.
14. McKeen, Laurence. “Acrylonitrile Butadiene Styrene.” NeuroImage, Academic Press,
2009, www.sciencedirect.com/topics/materials-science/acrylonitrile-butadiene-styrene.
15. “Nylon.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 3 Mar. 2017,
www.britannica.com/science/nylon.
16. “Process Analytics in Polypropylene (PP) Plants.” Industry USA Siemens, 2007,
www.industry.usa.siemens.com/automation/us/en/process-instrumentation-and-analytics/process-analytics/pa-case-studies/documents/cs_process_analytics_in_pp_plants.pdf.
17. “Processes, Stages, and Benefits of Plastic Recycling.” Plastic Recycling - Processes,
Stages, and Benefits, Compactor Management Company, www.norcalcompactors.net/processes-stages-benefits-plastic-recycling/.
18. “PTF: ENVIRONMENTAL IMPACTS.” Ecology Center,
ecologycenter.org/plastics/ptf/report3/.
19. “Rubik's Cube.” How Products Are Made,
www.madehow.com/Volume-7/Rubik-s-Cube.html.
20. “Rubik's Cube: How It's Made and Solution Clues.” Craftech Industries, 30 Oct. 2018,
www.craftechind.com/rubiks-cube-made-solution-clues/.
21. “Styrene.” Environmental Protection Agency,
www.epa.gov/sites/production/files/2016-09/documents/styrene.pdf.
22. Shearlock, Carolyn. “Silicone or Nylon Cooking Tools?” The Boat Galley, 14 Dec. 2012,
theboatgalley.com/silicone-or-nylon-cooking-tools/.
23. Sher, Stephen Tsung-Han. The New Durable Rubik's Cube. Indiana University, 9 Apr.
2014, http://homes.sice.indiana.edu/stsher/files/Rubiks_Cube.pdf.
24. Szaky, Tom. “The Many Challenges of Plastic Recycling.” Sustainablebrands.com, 22
Apr. 2015, www.sustainablebrands.com/news_and_views/waste_not/tom_szaky/many_challenges_plastic_recycling.
25. “THE PROBLEMS WITH PLASTICS.” Ecology Center, ecologycenter.org/plastics/
26. Thomas, G.P. “Recycling of Polypropylene (PP).” AZoCleantech.com,
AZoCleantech.com, 2 June 2016, www.azocleantech.com/article.aspx?ArticleID=240.
27. “Toxic Substances Portal - Acrylonitrile.” Centers for Disease Control and Prevention,
Centers for Disease Control and Prevention, 21 Jan. 2015, www.atsdr.cdc.gov/phs/phs.asp?id=445&tid=78.
28. “Toxicity of ABS Plastic in 3D Printing.” Whiteclouds 3D Printing, 2013,
ss.whiteclouds.com/blog/toxicity-abs-plastic-3d-printing.
29. Zafar, Salman. “Methods for Aluminium Recycling.” EcoMENA, 22 Feb. 2017,
www.ecomena.org/recycling-aluminium/.