Design Life-Cycle
assess.design.(don't)consume
Melody Naval Prado
Professor Cogdell
DES 40 Winter 2016
March 14, 2016
Carmex Ingredients
Carmex Medicated Lip Balm is a pharmaceutical product that is utilized to protect and soothe the lips from the environment that dry and crack them. [10] This product was invented in 1937 by Alfred Woelbing, who was in search for a remedy that would moisturize his lips. He manufactured the balm at home, which required using natural ingredients, and then packaged them in a jar. [17] The goal was not to find a way to produce the product at a sustainable rate, nor make the product biodegradable. Alfred was just looking for a way that the lip balm would be easy to produce and that was marketable. Despite the fact that by 1976 his products were made in factories, Carmex still attempts to make to all of its lip balms with natural ingredients. Today, there are now two Carmex factories, one located in Franklin, Wisconsin and the other in Atlanta, Georgia. All of the lip balms, whether in a tube, jar, or stick, resemble the original lip balm because they still contain some natural ingredients. They all contain four basic active ingredients, or in other words ingredients that will affect the lips, which are camphor, phenol, menthol, and salicylic acid. Carmex is made up of 1.7% camphor, 0.7% menthol, 0.4% phenol, and a very small amount of salicylic acid. [22] Each of these ingredients contribute to the characteristics that the lip balm contained in the jar presents. Carmex can aim to make its product environmentally friendly, starting from the location and the materials that it extracts its ingredients from.
It is unclear where Carmex got its ingredients, so it was necessary to make several assumptions. Camphor, phenol, and salicylic acid could have come from rosemary leaves and menthol from peppermint leaves. The other option is that camphor was drawn out from camphor trees, phenol from petroleum, menthol from peppermint leaves, and salicylic acid from wintergreen leaves. Both options contain some natural ingredients, which reflects how Carmex is trying to abide by the original natural ingredients.
Camphor is a solid and crystal substance that is extracted from camphor laurel trees. [18] It is not clear where Carmex gets their camphor from because camphor trees are found in Japan, Egypt, Taiwan, China, Madagascar, Canary Islands, southern Europe, West Texas, California, Georgia, Florida, and Argentina. [2,4,19] It is assumed that the trees come from Asia or from America. The reason why camphor can come from Asia is because they are native and predominant in Japan, China, and Taiwan. America has traded with Asia to obtain camphor from Japan or Taiwan. [2] It is also possible that Carmex's camphor is derived from America considering that it has several plantations of camphor laurel trees. If the trees were to come from the U.S., the transport would be less expensive and less time consuming. It would also be less wasteful because Japan and Taiwan withdraw their camphor from the trees' branches, roots, or trunk, while American plantations pull it out from the aged camphor trees' leaves or twigs. [2] Regardless their origin, these trees are a great source of camphor because they contains about 35% of camphor in them. [20] To get the camphor from the tree, its bark or leaves must go through steam distillation, which is a purification process using vapor. [5] The oils will come out, but will begin to crystallize. To change its crystal state, camphor oil will go through sublimation, which means that it will be modified. [4]
Camphor not only can come from camphor laurel trees, but from rosemary, a plant from the mint family. It contains 10-20% camphor, which is less than what the components of a laurel tree would have. [14] Although, it is smaller in mass and hence less wasteful than using an entire tree. To get camphor, its dry leaves would also have to be distilled. The rosemary leaves can come from Asia or the Mediterranean, since they are native in those places. [12] It is also possible that they receive it from America since the U.S. has rosemary plantations in Florida and Georgia. It would be environmentally friendly if the rosemary leaves came from the same country where the Carmex factories are located, the U.S.
If in fact camphor is obtained from rosemary leaves, then then phenol and salicylic acid wouldn't have to be added to Carmex. Rosemary has natural occurring phenol and high amounts of salicylic acid. [14,16] Considering that Carmex has a very small content of salicylic acid, then excess silicates would have to be removed.
Regardless the origin of camphor, it has the same features. Its properties are evident in the lip balm's characteristics. Camphor is crystalline and white, contributing to the white, clear color of the lip balm. [3,18] Camphor is waxy in its solid state, which causes Carmex to have a waxy texture and feel to it. Camphor has an aroma, making the lip balm have an accentuated scent. Camphor acts as a cooling agent, analgesic, and a mild local anesthetic as well in the lip balm since it can excite or desensitize sensory nerves. [9,18] The purpose is to cool any burning sensation and relieve any itching or pain. [5]
Another natural occurring ingredient in Carmex is menthol, which is similar to camphor in its technique of extraction and contributing characteristics. Menthol is a white solid that is extracted from the oils of the peppermint plant. [9] Menthol is extracted in the same form that camphor is extracted, through steam distillation. [21] It causes a cool feel on the lips because it activates the cool receptors. [18] This and the counterirritant effect is soothing when the lips are experiencing a burning sensation. The antipruritic, mild local anesthetic, analgesic properties of menthol help relieve pain and itching on the lips. [7,8] It is possible that it comes from the U.S., considering that West Texas holds a plantation of peppermint. This is positive because it is relatively close to the Carmex factories.
Salicylic acid is another natural occurring ingredient because it comes from wintergreen leaves. [22] It is a solid crystal the is derived from the distillation of the wintergreen leaves. [1] Salicylic acid has no odor and, like menthol and camphor, has a white color. It acts as an anti-infective agent and a keratolytic agent in Carmex, meaning that it exterminates germs and will cause the damaged top layer of the lips to fall off. [9,18]Once the epidermis of the lip is removed, the lip can absorb the soothing menthol and camphor. Wintergreen leaves are found in North America, which is close as for the imports of salicylic acid. [22]
Differing from the three natural ingredients, phenol is a substance that is extracted from petroleum. Petroleum is distilled because it contains cumene. Cumene is then oxidized, or added to oxygen, to make phenol. [18] Like camphor and menthol, it is clear, which causes the lip balm's white color to have a slight limpid appearance. Phenol also contributes its antipruritic, anesthetic, and disinfectant properties to the lip balm. The lips absorb phenol which helps eliminate germs and reduce the itching and burning. [9,18] Apart from its dubious origin, petroleum is taken from the U.S., which is convenient in distance.
Although it would seem unlikely that Carmex would get its phenol from petroleum since it is known for using only natural ingredients, it is more likely that its phenol does come from petroleum. An ingredient that I didn't mention, because it didn't form part of the four active ingredients of Carmex, is petrolatum. [9] This is a by-product of distilled petroleum, a method which would have been used to get phenol. [15] An ingredient from petroleum is far from being natural. If phenol is extracted from phenol, then maybe the phenol wasn't a natural occurrence from the rosemary leaves. This leads to the thought that maybe Alfred found it easy to use rosemary and peppermint leaves to make Carmex, but after his death Carmex utilized peppermint leaves, petroleum, wintergreen leaves, and camphor trees.
Although effective on the lips thanks to all of the ingredients, Carmex still has room to improve its product. The fact that the majority of the ingredients are natural benefits the lips, but not the environment. Carmex might need to make biodegradable or recyclable containers because what will be thrown away is the container, not the natural or biodegradable lip balm. To support the manufacture of Carmex, which is bought all over the world, the plant and petroleum resources could potentially be exploited. It is also a concern it the extraction of the resources have to travel a long distance to reach the Carmex factories. Carmex might need to look into what ingredients will need the least amount of energy input, but are still the most beneficial for the skin. Maybe they could turn to some harmless and energy efficient synthetic materials. As for the ingredients that have to be exported from far locations, Carmex can also think about obtaining its ingredients from the U.S., which is local. Carmex should consider changing their product to make it environmentally friendly.
Bibliography
1. Brown, William H. "Salicylic Acid." Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web. 14 Mar. 2016.
2. "Camphor." A Modern Herbal. N.p., n.d. Web. 14 Mar. 2016.
3. "Camphor." Camphor. N.p., n.d. Web. 14 Mar. 2016.
4. "Camphor." Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web. 14 Mar. 20, 2016
5. "CAMPHOR: Uses, Side Effects, Interactions and Warnings - WebMD." WebMD. WebMD, n.d. Web. 14 Mar. 2016.
6. "Camphor USP." Food and Cosmetics Toxicology 16 (1978): 665-71. Web
7. Eccles, R. "Menthol and related cooling compounds." Journal of Pharmacy and Pharmacology 46.8 (1994): 618-630.
8. Galeotti, Nicoletta, et al. "Menthol: a natural analgesic compound." Neuroscience letters 322.3 (2002): 145-148.
9. "Hardworking Medicated Lip Balm Ingredients | Carmex." My Carmex Hardworking Ingredients Comments. N.p., n.d. Web. 14 Mar. 2016.
10. Lane, Barry. "Lip balm composition." U.S. Patent No. 5,503,825. 2 Apr. 1996.
11. "Phenol." Phenol. N.p., n.d. Web. 03 Feb. 2016.
12. "Rosemary." Herbs2000.com. N.p., n.d. Web.
13. "Rosemary." University of Maryland Medical Center. N.p., n.d. Web. 14 Mar. 2016.
14. "Rosemary Uses, Benefits & Dosage - Drugs.com Herbal Database." Rosemary Uses, Benefits & Dosage - Drugs.com Herbal Database. N.p., n.d. Web. 14 Mar. 2016.
15. "Side Effects of Petrolatum." LIVESTRONG.COM. LIVESTRONG.COM, 11 Mar. 2011. Web. 14 Mar. 2016.
16. Swain AR, Dutton SP, Truswell AS. Salicylates in foods. J Am Diet Assoc. 1985 Aug;85(8):950-60.
17. "The Man behind Carmex." The Man behind Carmex. N.p., n.d. Web. 14 Mar. 2016.
18. The PubChem Project." Whats New in PubChem RSS. N.p., n.
19. University of Florida, IFAS Extension, Circular 1529, Invasive Species Management Plans for Florida, 2008
20. Volatile Components of Leaves, Stems and Bark of Cinnamonum Camphora Nees Et Ebermaier." Journal of Essential Oil Research 1995th ser. 7.3 (n.d.): n. pag. Web.
21. "What Is Menthol?" WiseGEEK. N.p., n.d. Web. 14 Mar. 2016.
22. Wikipedia. Wikimedia Foundation, n.d. Web. 14 Mar. 2016.
Ysabella Wood
DES 40A
Professor Cogdell
March 23, 2016
Life Cycle of Carmex: Energy
Lip balm has a relatively short history, only being first marketed in the 1880s in replacement of earwax for cracked lips. Carmex was one of the first products to be widely distributed for use for cold sores and chapped lips. Alfred Woelbing, the creator of Carmex and founder of Carma Labs. Inc., suffered from cold sores and since there were no remedies available he made one himself. Soon after in 1937 he began to make it at home and put it in the iconic jars to sell to pharmacies (Carmex). Eventually they caught on and he began receiving large orders for his lip balm; the high demand required him to move to a production facility in the 1950s. In the 1970s they moved to the current manufacturing facility in Franklin, WI (Carmex). It was about this time that Woelbing retired, as he was in his 70s, and his son took over for the company. From this point on the company expanded its products, adding squeezable tubes to the roster. By 1999, Carmex was the “#1 recommended over-the-counter lip balm year after year” (Carmex). In 2002, the company expanded its distribution area, selling to all 50 states, Canada, Europe, and Asia (Carmex). Currently, Alfred’s grandsons are the heads of the company and continue to expand the company's reach even farther. Due to the immense changes, the company has been consuming more materials, making more waste, and especially using more and more energy. Carmex lip balm itself is made of many things, but only four of the ingredients are active. Since technically the product could be made without many of those, I decided to focus only on the active ingredients. Those are camphor, menthol, salicylic acid, and phenol; while only making up less than 5% of the product, they are extremely energy intensive. Over the years, the company has made improvements to their process to be more ‘green’, but it proves to not be enough. Carmex Medicated Lip Balm, produced by Carma Labs Inc., aims to be an environmentally friendly product in the whole span of its lifecycle, but the energy consumption needed overall shows otherwise.
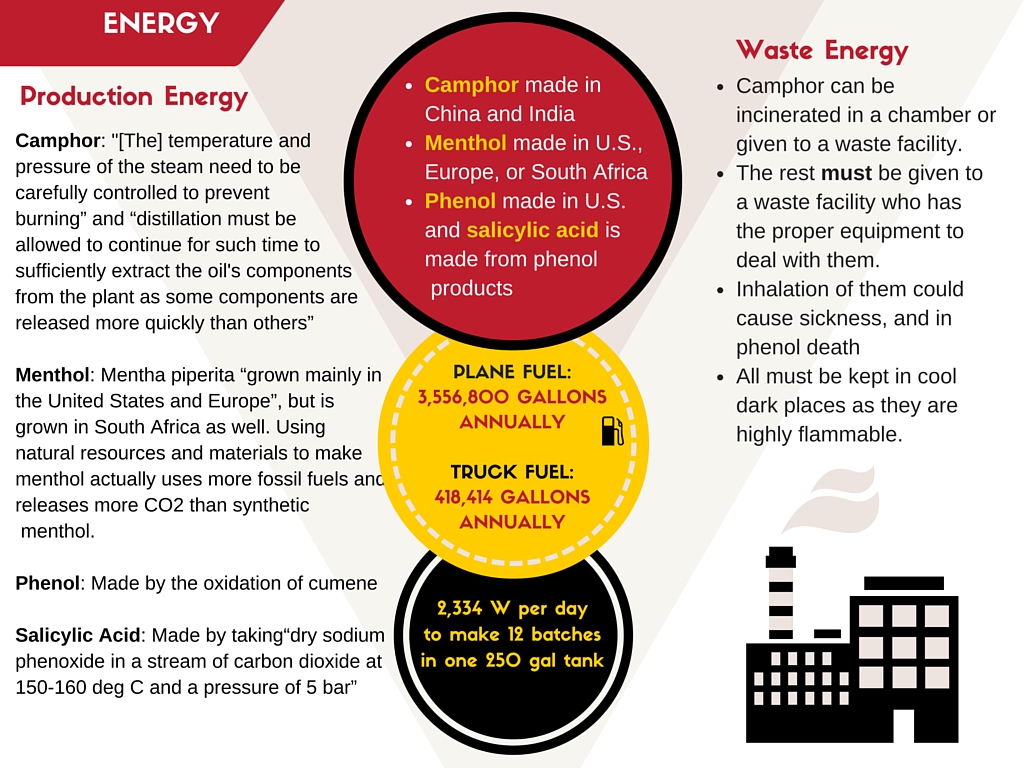
In terms of the materials used for the active ingredients, they are mostly synthetic. Camphor can be derived from the camphor tree, but the amount needed for all the reasons it is used is too high. Camphor is made synthetically made primarily in China, but some is produced in India (Synthetic Camphor). This means fuel consumption for whatever method used to send it to the U.S. Camphor “ [can] be obtained from steam distillation of parts of the camphor tree; however it is usually made from alpha-pinenevia camphene, to bornyl acetate, followed by saponification and oxidation” (Camphor | C10H16O). While it was difficult to find information on the exact process, and therefore the energy possibly associated, the information I could find showed the use of many chemicals. Most of them were taken from byproducts of processes for other products. One method is using steam distillation, as it is best for getting essential oils. It is a arguably laborious venture as “[the] temperature and pressure of the steam need to be carefully controlled to prevent burning” and “distillation must be allowed to continue for such time to sufficiently extract the oil's components from the plant as some components are released more quickly than others” (Steam). As Carma Labs claims to use menthol derived from peppermint oil, so I did not include the process for making synthetic menthol. Commonly, peppermint oil is produced from mentha piperita, which is “grown mainly in the United States and Europe”, but is grown in South Africa as well (Separation, Peppermint Production). While it is grown in these areas, peppermint oil itself is usually made in areas with cheap labor costs, China and India specifically (Peppermint Production). Using natural resources and materials to make menthol actually uses more fossil fuels and releases more CO2 than synthetic menthol. But due to its high menthol content (the highest of common mint plants), it is an extremely desirable product in the U.S. (Peppermint Production), and is a mass scale production. Phenol is another large component of the active ingredients and is usually synthetically created “by the oxidation of cumene” in the United States (Phenol | C6H6O). This method is used because the chemicals oxidized or chlorinated are cheap, and phenol is more valuable and harder to find naturally (Phenol | C6H6O). Another of the ingredients is salicylic acid, which is primarily sourced from phenol and phenol related products (Salicylic Acid | C7H6O3). The most common way of obtaining salicylic acid is through heating and combining “dry sodium phenoxide in a stream of carbon dioxide at 150-160 deg C and a pressure of 5 bar” (Salicylic Acid | C7H6O3). So while the exact information of how exactly the process for each of these chemicals works is not available, the general information of how they are made shows a high amount of energy. Some of the energy consumption comes from the energy needed to create the chemical itself, and the rest for other chemicals and materials needed to make camphor, menthol, salicylic acid, and phenol.
The type of energy needed for the manufacturing, processing and formulation of Carmex and its active ingredients is mostly consisting of electricity. Generally this electricity is used in the heating and mixing of all the ingredients to create the lip balm. There are quite a few machines used in the process, but “large mixing tanks of several hundred-gallon capacity and 10-quart pots (kettles)” are relevant to the active ingredients (Carma Laboratories, Inc.). Along with the other materials, camphor, menthol, phenol, and salicylic acid are heated up. Their melting points vary, with camphor at 177 °C, menthol at 40 °C, phenol at 40 °C, and salicylic acid at 158-160 °C (Material Safety Data Sheet: DL-Camphor, Menthols, Hazardous Substance , Salicylic Acid). First camphor and phenol are mixed together, meaning the temperature must be at least 177 °C, but not over 180 °C because that is the boiling point of phenol. Menthol and salicylic acid could both be added without worry to burning off the chemicals, so the minimum would still be 177 °C with maximum at 180 °C. Assuming that the tanks are about 250 gallons, and that the energy required to raise the temperature one degree celsius is the same as water, it would take 699999.00705 Joules over 1 hour to melt. This would be about 194.44416863 watts. If they have the facility open 12 hours a day that means 2,333.3300196 watts per day to make 12 batches in one tank; this is also assuming maximum efficiency. As there is no information as to how many days they are open a year, how many tanks they have, or other relevant information, I could not calculate the actual annual energy consumption of one tank. I estimated to the best of my ability and feel it is as accurate as it could be given the situation.
After the lip balm is made, it is then distributed all over the U.S. and the world. This requires truck, train or air transportation within the U.S. and Canada, but ships or airplanes must be used for destinations outside of North America. According to the United States Department of Transportation Bureau of Transportation Statistics, the amount of fuel consumed for international cargo airplanes is 5.9884 x 109 gallons a year in 2014 alone (RITA | BTS | Transtats). Carma Labs has a 23.5 million dollar revenue estimate; with products that average about $1.86 per individual container they produce about 1,344,086 containers of Carmex a year (Lip Care Products). From this point on I will assume that half of their products are shipped by air, and that cargo planes with Carmex lip balm in them only have said product. In this scenario the cargo plane is a Boeing 747, which holds about 48,445 gallons of fuel (747-400f). It uses approximately 3,600 gallons of fuel per hour, which means on a 10 hour flight it would use 36,000 gallons of fuel. I gave an extra 3 hours of ‘safety fuel’, which brings the total up to 46800 gallons (Hourly Fuel). I estimated the weight of each container to be 0.3333 oz and the volume to be 3.14 in3; each Boeing 747 with a Pratt & Whitney engine weighs 164,380 kg and can hold up to 295,740 kg (for landing weight), meaning it has the capacity for 131,360 kg for cargo (747-400f.). Though it can hold that much weight, the volume of the product does not allow for the full weight to be maximized. The lower cargo deck has 4631 ft3 available, and with each Carmex container being about 3.14 in3 that means each plane can hold 17,698.0892 containers per trip. As there are about 1,344,086 are made a year and I am estimating that about half are shipped by airplane, that means it is 672,043 containers. If you divide that by 17,698.0892 (the amount a cargo plane can hold), it shows that you would need 37.9726304, or 38, cargo plane shipments a year. If this is true, we can multiply the fuel consumption of one plane by 38 to get the total yearly amount; this amounts to 3,556,800 gallons of fuel per year for shipping by cargo airplane. As for in country, I am assuming that all shipments are done by trailer truck. In order to get an average distance traveled I took the distance from Franklin, WI to San Diego, CA and Franklin, WI to Miami Florida; this average was 1,790 miles. Multiply that by two to get the round trip, and it is 3,580 mi. Divide this by the average mpg that a trailer truck can hold, which is 5.75 mpg (Chapter 3). For one round trip it would take about 622.6 gallons of fuel. If a truck can hold 1,000 containers of Carmex, this would mean 672.043 trucks would be needed a year. This adds up to 418,413.972 gallons of fuel for trailer trucks annually round trip. Overall, the total amount of fuel needed to ship out the products all over the world would amount to 3,975,213.97 gallons annually. If the demand continues as it is, or gets even larger the amount of fuel needed will be overwhelming.
As for the waste management, there is not much information as to what it is they do. The Sigma Group gave a little bit of information on it, such as to the clean up of the residue left in tanks after a batch. Most of the “cleaning operations produce a waste water with high levels of emulsified oil and grease”, which was confirmed through both Carma Labs and Milwaukee Metropolitan Sewerage District (Carma Laboratories, Inc.). They created a new water treatment system that is supposedly better for the environment and more cost effective but there is no available evidence so far. According to the Wisconsin list of state solid waste facilities, there is one for ‘sludge’ and ‘tank sludge’ in Franklin (Solid Waste). I would assume all of the waste involved would fit under this category, or at least not in the others there. As for the individual active ingredients, they would have to be taken care of separately. They are all somewhat hazardous on their own, to varying degrees. Camphor must be incinerated “in a combustion chamber equipped in with an appropriate gas cleaning device” (Occupational ) or else it could cause irritation of they eyes, skin, mucous membranes, and could cause a cough (Camphor | C10H16O). Menthol is a bit more involved as “a licensed professional waste disposal service [should be called] dispose of this material” (Material Safety Data Sheet). Face masks such as the “full-face particle respirator type N100 (US)” and body suits along with gloves should be used, and disposed of when done (Material Safety Data Sheet). Salicylic acid is considered a hazardous waste and must be disposed of safely. It is only irritating to skin and lungs, but toxic if swallowed (Salicylic Acid). Phenol is a mutagen and should be treated as a possible carcinogen; it can cause very severe problems if ingested, inhaled, or touched. All must be kept in cool and dark areas, as they are all flammable and potentially dangerous (Hazardous Substance). All but camphor must be taken to another facility to be dealt with, and there is no information on the processes. As for use, there is no energy required. The product cannot be reused and the packaging should be recycled if possible. There is no maintenance of the product, as it is just something you use as is until you are done.
During my research, I realized how a product as simple as lip balm is not as simple as it seems. The amount of energy needed for the raw material acquisition, production/manufacturing, and transportation/distribution is tremendous. The lack of information released made it difficult to get an actual amount of energy for any of the steps of the life cycle. By being a private company, Carma Labs does not have to release all of their information, only what they choose. Based on the information I was able to find directly from the company itself and the numbers I calculated, the claim did not add up to the reality. The company claims to be a very ‘green’ and environmentally friendly. My research, while not completely precise, proves to some extent that their claims are not as true as they would like everyone to believe. This is not to say that that makes them a bad company; it just shows that they are not necessarily any better than the rest. While something may appear fine on the outside, it’s important to look into what you are buying and if it’s claims of good practices are valid. The more that people do this, the more the company will actually have to invest in making a better product all throughout its life cycle.
Bibliography
"Camphor | C10H16O." Camphor. Web. 02 Feb. 2016
"Carma Laboratories, Inc." The Sigma Group. The Sigma Group, 2014. Web. 03 Feb. 2016.
"Carmex Lip Care and Lip Balm Products : My Carmex." Carmex Lip Care. Carma Labs Inc, 2016. Web. 20 Feb. 2016.
Chapter 3 HEAVY TRUCKS. 2014 VEHICLE TECHNOLOGIES MARKET REPORT, 2014. PDF.
Hazardous Substance Fact Sheet: Phenol. New Jersey Department of Health, Jan. 2010. PDF.
"Hourly Fuel Consumption Calculator." Hourly Fuel Consumption Calculator. CSG, Computer Support Group. Web. 06 Mar. 2016.
Lip Care Products - Global Strategic Business Report. Rep. no. 338928. Global Industry Analysts, 2015. Print.
“l-Menthol | C10H20O.” I-Menthol. Web. 02 Feb. 2016.
Material Safety Data Sheet. Sigma-Aldrich, 26 Mar. 2014. PDF
"Material Safety Data Sheet: DL-Camphor." Chemistry Department Iowa State University. Fisher Scientific, 27 Aug. 2003. Web. 06 Mar. 2016.
Menthols. Paris: United Nations Environment Programme, August 2003. PDF.
Occupational Health Guideline for Camphor (Synthetic). Centers for Disease Control, Sept. 1978. PDF.
Peppermint Production: ESSENTIAL OIL CROPS Production Guidelines for Peppermint. Pretoria: Directorate Communication Services Department of Agriculture, Forestry and Fisheries, Feb. 2012. PDF.
“Phenol | C6H6O.” Phenol. Web. 02 Feb.2016.
"RITA | BTS | Transtats." RITA | BTS | Transtats. U.S. Department of Transportation (US DOT), 6 Mar. 2016. Web. 6 Mar. 2016.
Salicylic Acid. Anchorage: Health, Safety and Environmental Department, 27 Mar. 2012. PDF.
"Salicylic Acid | C7H6O3." Salicylic Acid. Web. 02 Feb. 2016.
Solid Waste Storage / Processing (Non-Landfill) Facilities Licensed in Wisconsin. State of Wisconsin Department of Natural Resources, 01 Dec. 2015. PDF.
"Steam Distillation." National Industrial Chemicals Notification and Assessment Scheme. Australian Government Department of Health, 2016. Web. 20 Feb. 2016.
"Synthetic Camphor Manufacturers." Synthetic Camphor Manufacturers. Global Manufacturers, 2016. Web. 20 Feb. 2016.
747-400f. Chicago: Boeing, May 2010. PDF.
Wing Yi Truong
DES 40A
03- 06- 2016
Waste and Emission of Carmex Lip Care
Raw Material Acquisition
The Carmex is one of The oldest well-known lip care, lip balm company in the United States, it offers lip balms in different proportions, such as in jars, tubes, and lipsticks. Carmex claimed that their “natural” lip balm formula helps remove cracked and peeling skin, and allow soothing and healing dry lip, and the menthol and camphor ingredients provide a cool and refreshing tingle. (Carmex) The natural lip balm formula based on four main raw materials, which are Salicylic Acid, Phenol, Menthol and Camphor. Regarding to those four materials, they are considered as hazardous materials, which are Eco-toxicity to human and to our environment. For the acquisition of those four raw materials, the synthesis salicylic acid is extracted from aspirin, in the manufacture process if aspirin and salicylates may release various stream waste to the environment, its former United States use as a fungicide release directly to the environment and its distributed all round plants. Salicylic Acid is also a synthesized substance from Phenol. There is water used for extraction. During the batch process, the sodium Salicylic dissolved in water and pumped to a treatment tank in order to get the pure and pharmaceutical level of Salicylic Acid. It's also carries with byproduct and it will have removed by wastewater from the extraction process. (Toxnet) Secondly, the Camphor production uses as a flavor and redolence composition, the major ingredient of Camphor is extracted from camphor trees, and the natural Camphor is acquired by purification from the Cinnamomum and Laurus Camphora, which from China, Japan, and India. Camphor oil is generated as a byproduct in the extraction of Camphor. The third raw material is Menthol, it is extracted from corn mint and peppermint, it also can have extracted from synthetically produced, the main source of the pollution for natural Menthol is to cool the oil in order to crystallize the menthol and purification of the oil, it’s made in the United States, Europe, and Africa. The last raw material is Phenol, Phenol is considered as a highly corrosive hazardous substance to human and animal skin. According to U.S. National Library of Medicine, Phenol can be produced naturally and synthesized. Naturally, it is a constituent of coal tar and creosote, but nowadays, the Phenol usually made out of oxidation of Cumene in the United States. (Toxnet)
Manufacturing, processing and formulation:
Carmex is a worldwide lip care company produces different kinds of lip care and skin products, such as lip balms in jars or tubes and sticks, which sell their product in the United States and globally, Carmex does not have any specialty store, they usually offer their lip balm product through drug stores, mass merchant retailers, convenience stores, and online store in the United States all 50 states, included Canada, Australia, and Europe. (Carmex) Carmex has two major factories in order to manufacture lip care products, which is Carma Laboratories and Carmex Lip Factory. Carma Laboratories are located in Franklin, Wisconsin, which at in the greater Milwaukee area. it’s also the headquarter of the company. The Carmex Lip Factory is located in the Southside of Atlanta in the state of Georgia. The Carma Laboratories have never released any information on the manufacturing process. (Georgia Economy Profile) Due to this reason, our group could not find any detail information about the factory conditions with regards to air and water quality in both Carmex factories. However, we found an Environmental engineering company that providing sustainable solutions for other companies, the Sigma Group is located at Milwaukee, one of their projects is helping Carma Laboratories, Inc. on developing equipment for resolve the wastewater treatment system. The Sigma Group stated that in the manufacturing process of Carma Laboratories, Inc., it needed several hundred- gallon mixing tanks to mix different compounds, after the mixing process, it required a massive amount of water to remove Carmex residue remains in the tanks. it produces high levels of emulsified oil and grease in the cleaning operations, the Sigma Group has also sampled the wastewater in the Milwaukee Metropolitan Sewerage District, the wastewater has discharged up to 2,000 milligrams per liter of emulsified of oil and grease, which is much higher than the discharge limit of 300 milligrams per liter. (Sigma) Based on the four raw material Hazardous Material Safety Data Sheet, I found that Salicylic Acid could be released to the aquatic environment in wastewater discharge from industrial. According to U.S. Environmental Protection Agency (EPA) survey of wastewaters of 46 industry categories for a total of 129 priority pollutants from 1976, 2 of 21 industrial categories of wastewater effluents had detected Salicylic Acid. Moreover, Salicylic Acid decomposes carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide during the manufacturing process. Based on the Salicylic Acid document form U.S. National Library of Medicine, Salicylic Acid had detected in the wastewater effluent of a mill in Georgia, which is one of the states of Carmex factories. the next raw material- Camphor, it also decomposes carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide during the manufacturing process. the concentration of Camphor was also detected from the effluent of two unbleached treated kraft mills in Georgia. For the Phenol, after it heated up to boiling point for approximately 30 minutes, there is byproducts may have generated during its oxidation in many industrial wastewaters.
Distribution and transportation
In the distribution and transportation process, Camphor is mainly shipped from China and India, Menthol is shipped from Europe and South Africa, and some of it is made in the United States, Camphor and Menthol both components needed more energy to import. During the making of chemicals, transportation, and distribution, there are 38 cargo plane shipments being exported to other countries. Carmex produced 292,953.868 pounds of Carbon dioxide (CO2) annually. All the raw material being ship from China and India by cargo plane, and after packaging, the Carmex lip product will ship to different retail stores and globally. It will produce sulfur dioxide and nitrogen oxides in the air transportation process. (ECONOMIC PROFILE City of Franklin, WI)
Use, re- use and maintenance
Salicylic Acid, Camphor, Menthol, and Phenol are highly dangerous substances to the environment, the environmental effect that comes in the use of Salicylic Acid in bodily, and toxic effects to human bodies lead to respiratory alkalosis, which means the amount of acids and bases human bodies are unbalance on the pH scale. It will cause the effects of nausea, vomiting, epigastric discomfort, tinnitus, loss of hearing, sweating, flushing. When salicylic acid released to air, it will exist in the vapor to degrade in the atmosphere by reaction with photochemically- produced hydroxyl radicals; when it's released to soil, this compound will exist primarily in the anion form in the environment and soil will contain organic carbon and clay than their neutral counterparts, volatilization from moist soil surfaces is not expected to be and important fate process because anions does not volatilize. Salicylic acid is unlikely to volatilize from dry soil surfaces based upon its vapor pressure. For the second material- Camphor, the U.S. Food and Drug Administration stated that Camphor was “Not Classifiable as a Human Carcinogen,” but there were Camphor poisoning case of symptoms in adults and children such as stomach ache, disgust, vomiting, diarrhea, and anxiety. If Camphor exposes to respiratory or lung in long- term, it will involve difficult breathing and irritate to eyes and a risk of loss of smell. When Camphor released into the atmosphere, it will evaporate into the atmosphere and it’s reactive in the air for about three and a half days. When Camphor released in soil, Camphor only expected to evaporate from dry soil. For the third material- Menthol, it usually used for cooling and mint added flavoring, and it’s one of the substances added into confectionery products, liqueurs, chewing gums, and toothpastes, and cosmetics, but it had reported the use of Menthol will cause sensitivity reactions in adults and infants, the Menthol cause severe damage to the mucous membranes when irritant in human skin. On the other hand, when Menthol release of water or oil, it will not fasten or suspended in soil or water, which move into air from water and moist soil surface and it won’t break down by any micro-organisms. The last material- Phenol, it’s contains the most highly toxic in four of the Carmex components. When it is exposed to the human body, it initiates shock, delirium, coma, pulmonary distress, phenolic breath, and death. and it will be cause oral lethal dose to human. When it releases to air, it remains in the atmosphere as a vapor. It also not expected to volatilize on the water surfaces. (Toxnet)
Recycle
Carmex provides Money back guarantee for only users in the United States, and there is a limitation of required per buyer and one per household when purchase up to $16.99 U.S dollars. Based on the Material Safety Data Sheet from United States Department of Labor, the product of Carmex Lip Balm: original Stick is less than 1% of synthetic Camphor and is not used in hazardous concentration. It may have disposed of in landfill, but it causes pollution and the hazardous chemical product will become a health hazard. Secondly, the product of Carmex is in emulsified form, substance, in order to wash off the oil and grease, it often uses wastewater to operate the treatment method, because it is simple to operate, save costs, and efficient on the discharge of contaminated wastewater. (How to Remove Emulsified Oil from Wastewater with Organoclays) Particularly, Salicylic Acid may have released to the environment in wastewater discharges from industries. In addition, Carmex will also end up in incineration. Based on the Guidance on Landfill and Incinerator Ban Enforcement in Wisconsin, Wisconsin required at least the minimum of 5,000 tons of waste, in order to receive the landfill operation and incinerate operation. In the incinerate operation, the incinerator has natural gas to heat the temperature up to 1400 degrees Fahrenheit to melt down waste, the incineration process requires the second incineration in order to eradicate the pollutants of flue gas going into the atmosphere, which needed even more natural gas in the operation, because in the incineration operation release a huge amount of Furans, Dioxins, and Carbon Dioxide (CO2). (Wisconsin Department of Natural Resources)
Waste Management:
For the Salicylic Acid, it is determined a discarded chemical is classified as a hazardous waste, if pulling Salicylic Acid into the ground, it will have biosynthesized inside plants and decaying plants, in the long term release of Salicylic Acid, it will reduce the foreign body response in mice. When pulling Camphor into the ground, it will have reached the biodegradation in soil in 4 weeks, and the concentration of Camphor was found in 60 municipal landfills in the United States. (Toxnet) The disposal method of Menthol will impact on the quality of air, soil, and water, and effects on animal, a marine and plant The high concentration of Phenol was detected in 9 groundwater wells in Wisconsin after a Phenol spill, which is happening in the main factory location of Carmex. The natural pollution sources from Phenol is existed in animals, and other domestic fowls. In the other hand, the operation of incineration spends a great amount of fuel to dissolve the material with the combustion in a chemical incinerator, and also used in afterburner of incineration. (U.S. Environmental Protection Agency)
Work Cited
"Incinerators." U.S. Environmental Protection Agency. Web. 12 Mar. 2016.
"Mineralization Improvement of Phenol Aqueous Solutions through Heterogeneous Catalytic Ozonation." - Beltrán. Web. 07 Mar. 2016.
Meade, Richard K. McCormack, Harry. Clark, Laurance T. Sclater, Alexander G. Lamborn, Lloyd. “Chemical Age.” McCready Publishing Company. 1915. Web. 07 Mar. 2016
"Solid Waste Storage / Processing (Non-Landfill) Facilities Licensed in Wisconsin." State of Wisconsin Department of Natural Resources, 01 Dec. 2015. Web. 8 Mar. 2016.
"Ban on Camphor Use in Temples to Be Strictly Enforced." The Hindu. 2014. Web. 09 Mar. 2016.
"How to Remove Emulsified Oil from Wastewater with Organoclays." How to Remove Emulsified Oil from Wastewater with Organoclays. Web. 12 Mar. 2016.
"Guidance on Landfill and Incinerator Ban Enforcement." Wisconsin Department of Natural Resources. Web. 12 Mar. 2016.
"Camphor Material Safety Data Sheet." Regentproducts. American Consumer Porducts. Web. 7 Mar. 2016.
"Georgia Economy Profile 2014." Georgia Economy Profile 2014. Web. 04 Mar. 2016.
"Salicylic Acid Material Safety Data Sheet." Chemistry Material Safety Data Sheets. Iowa State University, 02 Aug. 2000. Web. 07 Mar. 2016.
"L-Menthol Pharma and L-Menthol Flakes Pharma." Pharma- Ingredients. BASF The Chemical Company, Jan. 2013. Web. 6 Mar. 2016.
"Carma Laboratories, Inc." The Sigma Group. The Sigma Group, 2014. Web. 03 Feb. 2016.
ECONOMIC PROFILE City of Franklin, WI. Franklin: The City of Franklin Department of City Development, Mar. 2012. PDF.
"FAQ - Our Medicated Lip Treatment & Products | Carmex." My Carmex FAQs Comments. Carma Labs Inc., 2015. Web. 02 Feb. 2016.
"Carmex Lip Balm & Skin Care History - Explore Our Story." My Carmex Carmex History Comments. Web. 12 Mar. 2016.
"Camphor." TOXNET. U.S. National Library of Medicine. Web. 12 Mar. 2016.
"Phenol." TOXNET. U.S. National Library of Medicine. Web. 12 Mar. 2016.
"Salicylic Acid." TOXNET. U.S. National Library of Medicine. Web. 12 Mar. 2016.
"Menthol." TOXNET. U.S. National Library of Medicine. Web. 12 Mar. 2016.