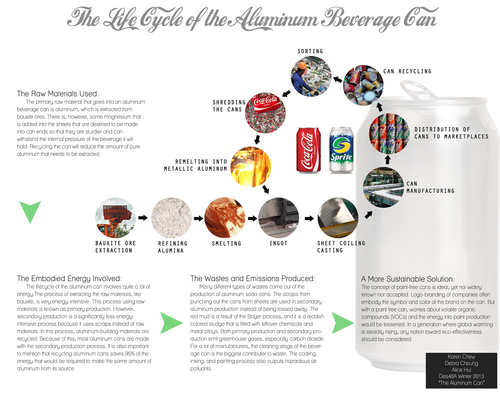
Design Life-Cycle
assess.design.(don't)consume
Karen Chew
Professor Cogdell
DES 40A
27 February 2013
Manufacturing the Aluminum Beverage Can: Raw Materials
Aluminum beverage cans are every day commodities that people come across on a regular basis, whether it’s while they are grocery shopping, taking their lunch break, or pondering in front of a vending machine. But few would put any thought into wondering how exactly the can in which holds the beverage they drink is made, or the raw materials going into and coming out of these aluminum cans. While there are chemicals and some alloys that enter or are byproducts of the process the manufacturing of the aluminum beverage can, bauxite ores are the main raw material that goes into this process, and recycled aluminum is the raw material that is extracted from the recycling process to be put back into the manufacturing process to decrease the amount of bauxite ores that needs to be mined.
Because aluminum is used in a lot of products, it did not take long to research how aluminum is processed into useable metal. Aluminum is extracted from bauxite ores, more specifically the minerals gibbsite, böhmite and diaspora, and is considered the most abundant metal existing in the Earth’s crust. It is generally mined overseas due to the fact bauxite reserves are mostly found in the tropical and subtropical regions of the world before it is imported into the United States. Prior to mining, the land is deforested and cleared while saplings and seeds are collected so that the land may be revegetated after its been depleted of bauxite.[1] Once, imported into the US, the bauxite undergoes the Bayer process to extract the aluminum from the ore.
The Bayer process was first invented and patented by Karl Josef Bayer, an Austrian chemist in 1887.[2] During research, I came across conflicting information, in which one of my sources claimed a man named Henry Bayer was the inventor of the Bayer process, while another source claimed it was Karl Josef Bayer. After consulting with several other resources, I came to the conclusion that the source sponsored by a trusted organization had the correct information, as no information for a “Henry Bayer” was found that was significant to the Bayer process of making pure alumina. The Bayer process is still the primary method used to extract alumina, which is also known as aluminum oxide, from bauxite today. Bauxite, after it has been washed, is passed through screens are crushed so that the ore particles are now a uniform size. It is then fed into grinding mills with lime and caustic soda, or sodium hydroxide, that comes from the precipitation stage of the Bayer process to be mixed at a high temperature and pressure to produce a mixture called “slurry”. The slurry consists of sodium aluminate as well as undissolved bauxite residues, including high concentrations of iron oxide, titanium dioxide and silicon oxide; the byproduct often referred to as “red mud”.[3]
The slurry is then pumped into a digester, where more caustic solution is added to help dissolve the sodium aluminate into the solution at a high temperature while undesired and undissolved residues are periodically cleaned out of the digester. Depending on the composition of the bauxite, it is either heated at 145°C (gibbsite) or 200° to 280°C (böhmite) to dissolve the sodium aluminate, before it is cooled in flash tanks at 106°C to reduce pressure and temperature, forming steam that is used to preheat the slurry and potentially saving energy usage.[4] The solution is then directed into settling tanks, where the red mud that is in the slurry is allowed to separate from the sodium aluminate and caustic soda concentrate and settle on the bottom of the tank; often chemical additives are added to the solution to help the sediment process.[5] The red mud is then transferred into washing tanks to recover more caustic soda before it is removed as a waste byproduct of the Bayer process. The concentrate, on the other hand, is processed through a series of filters to remove any remaining undissolved bauxite residue from the concentrate before it is pumped into the precipitators. There, fine particles of alumina called alumina hydrate are added to begin the precipitation of pure alumina particles as it cools. Once the alumina crystals settle onto the bottom of the tank, it is transferred and filtered again to wash away impurities and remove moisture before being moved into the calcining kilns. There the crystals are heated at temperatures as high as 1100°C while it is slowly rotated to further remove moisture before being cooled down; the result of this process is pure alumina in the form of white powder, which is, then, ready to be shipped of to be smelted into metal.[6]
In order to turn the alumina into metallic aluminum that is used to create the structure of the aluminum can, the fine powder undergoes the Hall- Héroult process, which is founded by the American chemist Charles Martin Hall and Frenchman Paul Héroult in 1886. The alumina, or aluminum oxide, is mixed with molten cryolite, a sodium aluminum fluoride mineral, to dissolve the alumina and separate the oxygen from it as direct electrical current (DC) is run through it, producing metallic aluminum and carbon dioxide gas.[7] Then, a small amount of other metals, such as magnesium, are added to the aluminum to strengthen the metal and resist corrosion better before it is cast into aluminum ingots that are rolled into long sheets and coiled and shipped to the can manufacturing plants.[8]
Once at the manufacturing plants, the aluminum coils are unrolled and lubricated so that the aluminum flows more smoothly as it is formed into a can. The aluminum sheet is then fed into the cupping press, which cuts out shallow circular cups before being drawn higher into a cup and ironed to add thickness to the walls in an ironing press.[9] The tops are then trimmed so that each can has a uniform height as well as a uniform width. The punctured scrap aluminum from this procedure is removed and recycled.[10] The can cut outs then undergo a cycle of cleaning and rinsing of the outer surface before it is dried and continues on their journey to be inked. The cans are rolled against a rubber cylinder that prints six to eight colors onto the can simultaneously as it spins before a varnish is applied to keep the can shiny and protect the paint. These cans then enter a baking oven to dry and secure the paint from chipping before a protective coating is sprayed onto the inside of the can to ensure that beverage that is to be contained in the can would never touch the metal before it is baked once again to seal the coating.[11] The top of the can—the neck—is narrowed in preparation of being sealed and the bottom of the can domed to maintain strength that is needed to withstand the internal pressure of the can after the drink is added. All cans are then processed through a light tester that is able to detect holes smaller than human hair to ensure there would be no leaks before they are palletized and shipped off to the customers—the beverage companies—to be filled with drinks.[12]
The lid of the cans, known as can ends, are made in a similar process, in which the aluminum sheet is uncoiled and lubricated before punched out as circular shells. These then go through a high precision press that is responsible rivet making, scoring and tabbing. Before the easy-open tab is attached onto the end, a sealant is coated all over so that none of the metal would touch the drink; when everything is done, they are bagged and shipped separately to the beverage companies.[13]
Now, to backtrack a little on the aluminum beverage can’s life cycle, I wanted to do more research on the way the ink and decoration of a beverage can is applied to evaluate whether or not there were some environmental effects in this process. This information proved to be harder to find than the aluminum-extracting process and the can-making process part of an aluminum beverage can’s life cycle; it didn’t help that US Patents are often densely written, making it harder to decipher whether I had found the relevant information I needed or if the patent was explaining some other process that was entirely irrelevant to this. However, after consulting and comparing several sources, I have discovered that there is potentially an environmental impact on the way the cans are printed upon.
According to the U.S. Environmental Protection Agency, many coatings—this includes inks, varnishes and base/rim coats—used by the beverage can industries are heavily concentrated with solvents, which resulted in large amount of volatile organic compounds (VOC’s) being emitted into the air that were harmful and potentially carcinogenic to the human body. Since the 1970s, clean air regulations have been passed demanding that for lower VOC content in the coatings, which led to the now widely used waterborne-based coatings used by beverage can companies.[14] The waterborne-based coating, made of polymer or resin base, water and organic solvent, use the same application equipment as the conventional solventborne coatings, but contain a significantly lower amount of VOC, at 1.4 to 3.6 lb VOC/lb gal coating versus the solventborne’s 4.0 to 6.6 lb VOC/lb gal coating.[15]
In 1977, Adolf Coors Company, now known as Coors Brewing Company, became the first and, still is, only beverage can company to use the UV curing method in the United States, with further innovations being added in 1986.[16] The process of in which the ink is applied is generally the same as that of the conventional solventborne coating method, except an ultraviolet photoreactive overvarnish layer is sprayed on top of the decorative layer (in which the ink also contains this UV photoreactive compound) before it is baked in a UV-radiated oven. The layers’ UV photoreactive compounds are cured in response to the UV radiation—it does not dry simply by air.[17] The UV baking system requires less energy than the convention oven that is heated by gas and is very efficient, as it is able to cure a can in 500 milliseconds, which increases product line speed,[18] and also emits less than 0.01 lb VOC/gal coating.[19] Unfortunately, the UV coating method is still not widely amongst the US’s beverage can companies.
Aluminum beverage cans are not only considered the most common beverage package, but also the most recycled consumer packing as well as the most valuable container to be recycled.[20] Since aluminum is a sustainable metal, it can be recycled over and over again, and beverage can companies have discovered that by reusing the recycled aluminum, they save 95% of the energy that it would normally take to make a can out of pure extracted aluminum.[21] For this very reason, many of these companies have been buying back as much of the recycled aluminum as possible so that they don’t have to spend nearly as much money and energy mining for bauxite ores. According to an analysis done in 2010, aluminum can recycling rate has reached 58.1%,[22] and an empty can generally can be recycled and make it back on the grocery shelf in as little as sixty days.[23]
Aluminum cans that are recycled are transported to smelting facilities, where they undergo tests for moisture content and quality of metal; those that pass are then shredded before heated to de-lacquer the paints and other materials on the can. Then, they are heated again at 1400°F (or 650°C) to be melted into molten metal that is poured into molds to form ingots. The aluminum ingots are then flattened into coils and then shipped to the beverage can companies to restart the cycle of being made into a can.[24]
While this all seems like the perfect solution to reducing the amount of pure aluminum used to make cans, the recycling process is actually a little more complicated than it appears. The aluminum sheets used for can ends generally are mixed with magnesium to help it withstand the bending force on the metal when it is opened; this means that when the cans are melted down into molten metal, it is actually an alloy that is not fit to be made into the aluminum sides of the can nor the sturdier can ends. In order to make it suitable for either of these types of sheets, either pure aluminum is added into the mixture to dilute the magnesium concentration and reduce the metal’s stiffness, making it suitable for can bodies, or more magnesium is added into the mixture so that it is suitable for making can ends.[25] But because the recycled aluminum method of saving energy during the can-making process has already proven to be very efficient, Novelis, the largest American supplier of aluminum sheets, has made it their goal to not only increase the recycling rate from 50% to 80% by 2020, but to also find a more efficient way to process the aluminum-magnesium alloy resulting from recycling beverage cans.[26]
Although there are chemical compounds, such as caustic soda and molten cryolite, that are added into the lifecycle of an aluminum beverage can, none of these are raw materials extracted from a natural resource, nor are do any of these appear within the final product of metallic aluminum. Cryolite, however, is a rare natural-occurring mineral that is used in the process of electrolysis of alumina to make metallic aluminum. But since it is hard to find, sodium aluminum fluoride is often synthetically made to substitute for the uncommon mineral.[27] Ultimately, the bauxite ores that aluminum is extracted from, along with the small amount of magnesium used in can ends, are the only raw materials that go into the aluminum can making process, and because it is a sustainable metal, aluminum is also the raw material that comes out of the recycling process of this entire process.
Bibliography
[1] "Mining Process." Aluminum for Future Generations. The International Aluminum Institute, n.d. Web. 3 Mar. 2013. <http://bauxite.world-aluminium.org/mining/process.html>.
[2] "Refining Process." Aluminum for Future Generations. The International Aluminum Institute, n.d. Web. 3 Mar. 2013. <http://bauxite.world-aluminium.org/mining/process.html>.
[3] "The Aluminum Page: How Aluminum is Produced." Rockman's Rocks, Minerals and Fossils. N.p., 16 May 1999. Web. 1 Mar. 2013. <http://www.rocksandminerals.com/aluminum/process.htm>.
[4] "Refining Process." Aluminum for Future Generations. The International Aluminum Institute, n.d. Web. 3 Mar. 2013. <http://bauxite.world-aluminium.org/mining/process.html>.
[5] Ibid.
[6]"The Aluminum Page: How Aluminum is Produced." Rockman's Rocks, Minerals and Fossils. N.p., 16 May 1999. Web. 1 Mar. 2013. <http://www.rocksandminerals.com/aluminum/process.htm>.
[7] "The Aluminum Page: How Aluminum is Produced." Rockman's Rocks, Minerals and Fossils. N.p., 16 May 1999. Web. 1 Mar. 2013. <http://www.rocksandminerals.com/aluminum/process.htm>.
[8] "About Alcoa Packaging: How Aluminum is Made." Alcoa RPD. Alcoa , n.d. Web. 27 Feb. 2013. <http://www.alcoa.com/rigid_packaging/en/about/making_cans.asp >
[9] "How Are Aluminum Cans Made?." Earth911. Earth911.com, Inc. , n.d. Web. 1 Mar. 2013. <http://earth911.com/recycling/metal/aluminum-can/how-are-aluminum-cans-made/>.
[10]"About Alcoa Packaging: How Aluminum is Made." Alcoa RPD. Alcoa , n.d. Web. 27 Feb. 2013. <http://www.alcoa.com/rigid_packaging/en/about/making_cans.asp >
[11]Ibid.
[12]How Are Aluminum Cans Made?." Earth911. Earth911.com, Inc. , n.d. Web. 1 Mar. 2013. <http://earth911.com/recycling/metal/aluminum-can/how-are-aluminum-cans-made/>.
[13] Ibid.
[14] "Preliminary Industry Characterization: Metal Can Manufacturing--Surface Coating." U.S. Environmental Protection Agency. U.S. Environmental Protection Agency, Feb. 1998. Web. 6 Mar. 2013. <http://www.epa.gov/ttn/atw/coat/mcan/pic-can.pdf>.
[15]bid.
[16] "UV Curing of Coatings on Metals." Pollution Prevention Resource Exchange. EPRI Center of Material Fabrication, Aug. 1991. Web. 6 Mar. 2013. <http://infohouse.p2ric.org/ref/26/25750.pdf>.
[17] Schultz, Robert H., “Device and Method for Uniformly Curing UV Photoreactive Overvarnish Layers”. Patent 4,503,086. 5 March 2985.
[18] UV Curing of Coatings on Metals." Pollution Prevention Resource Exchange. EPRI Center of Material Fabrication, Aug. 1991. Web. 6 Mar. 2013. <http://infohouse.p2ric.org/ref/26/25750.pdf>.
[19] "Preliminary Industry Characterization: Metal Can Manufacturing--Surface Coating." U.S. Environmental Protection Agency. U.S. Environmental Protection Agency, Feb. 1998. Web. 6 Mar. 2013. <http://www.epa.gov/ttn/atw/coat/mcan/pic-can.pdf>.
[20] "Packing Materials: Aluminum." American Beverage Association. American Beverage Association, n.d. Web. 7 Mar. 2013. <http://www.ameribev.org/minisites/recycling/packaging/materials.php>.
[21] "Facts about Aluminum Recycling." Earth911. Earth911.com, Inc. , n.d. Web. 7 Mar. 2013. <http://earth911.com/recycling/metal/aluminum-can/facts-about-aluminum-recycling/>.
[22] "The Infinitely Recyclable Aluminum Can." Aluminum: The Aluminum Can. The Aluminum Association, 2008. Web. 7 Mar. 2013. <http://www.aluminum.org/Content/NavigationMenu/TheIndustry/PackagingConsumerProductMarket/Can/default.htm#Aluminum Can Recycling>.
[23] "Facts about Aluminum Recycling." Earth911. Earth911.com, Inc. , n.d. Web. 7 Mar. 2013. <http://earth911.com/recycling/metal/aluminum-can/facts-about-aluminum-recycling/>.
[24]"A Day in the Life of Aluminum Cans." American Beverage Association. American Beverage Association, n.d. Web. 7 Mar. 2013. <http://www.ameribev.org/minisites/recycling/a-day-in-the-life/index.php>.
[25]Wald, Matthew L. "Towards a Greener Soda Can." Green: A Blog about Energy and the Environment. The New York Times, 12 June 2012. Web. 7 Mar. 2013. <http://green.blogs.nytimes.com/2012/06/12/toward-a-greener-soda-can/>.
[26]Ibid.
[27] "The Mineral Cryolite." Amethyst Galleries' Mineral Gallery. Amethyst Galleries, Inc. , n.d. Web. 9 Mar. 2013. <http://www.galleries.com/Cryolite>.
Alice Hui
Professor Cogdell
DES40A
13 March 2013
Embodied Energy in the Process of Aluminum Soda Can Production
In any sort of design production life cycle, there are several important inputs involved that make the process successful. These inputs include the raw materials, the embodied energy, and wastes and emissions. Since my group has decided to research on the production life cycle of an aluminum soda can, we each dove into a specific area to have a better focus and understanding of the process of production. I am focusing my area of research on the embodied energy. Through much research and class discussions, I’ve discovered that the amount of energy it takes to produce aluminum soda cans is actually quite large. Furthermore, I’ve learned that it is important to take the life cycle approach as an opportunity to make well-informed choices towards the community and environment.
“The impacts of all life cycle stages need to be considered comprehensively when taking informed decisions on production and consumption patterns, policies and management strategies” (UNEP, 3). According to UNEP, the life cycle approach gives people the opportunity to make well-informed choices as individuals and for companies they work for. In addition, it helps find ways to generate energy we need without depleting the source of that energy and without releasing harmful emissions in the atmosphere. There are many steps that must be taken in the life cycle of the aluminum can production. Throughout these steps, energy will inevitably be used— energy that comes from the systems that produce, distribute, disassemble, and recycle the product. The aluminum industry, however, has taken “consistent and direct action to reduce the amount of material and energy used in association with its production of the aluminum beverage cans—both by continually lightening the gauge used in the manufacture of aluminum beverage cans and by increasing the recycled content used in each can” (The Aluminum Association). Energy usage has definitely been an issue in the past, so with aluminum being the second most used metal after steel for modern societies, it would be helpful to understand how much energy is used in the life cycle of the aluminum can as a way find approaches to reducing energy usage.
“The production of aluminum from bauxite requires much more energy than many other metals and causes large amounts of greenhouse gases (GHG) emissions” (Liu and Muller, 108). In fact, aluminum production is actually responsible for much energy usage at least 1% of the annual GHG emissions. To understand why this is the case, I studied Liu and Muller’s article, which reviews the life cycle description of an aluminum product system. There are a several important processes in the full life cycle of the aluminum product system. This includes the following:
Mining and Production
Aluminum is produced from bauxite ore, which is primary, or from scrap, which is secondary. Primary production involves making aluminum from raw material while secondary production involves recycling aluminum scrap to form new products. Primary aluminum is produced through the well-established Bayer process for alumina from open-pit mined bauxite and follows with Hall-Heroult electrolysis (Liu and Muller, 109). Furthermore, there are molten aluminum from the smelters, or electrolyzing baths, that are alloyed, cleaned, and cast into different kind of ingots. Secondary aluminum, on the other hand, is produced from scrap in refiners and remelters, which produce casting alloys and deoxidation aluminum primarily from post-consumer scraps. Overall, primary production is highly energy intensive while secondary production is a significantly less energy-intensive process.
The process of producing the pure metal from its most basic state (Bauxite) requires a great amount of energy. “The basic measure of energy used to make a material is the total amount of energy consumed by all of the processes associated with its production; it is usually a tally of BTUs needed to (in the case of aluminum) mine, process, and form the base material into the finished project” (Streamline). BTU is British thermal unit, which is a traditional unit of energy equal to about 1055 joules. According to Streamline, it actually takes about 200 million BTUs to make one ton of pure aluminum material, which is 7 times the amount of energy that is required for making just one ton of new steel. Moreover, the primary production of aluminum is produced through electrolysis, a method that uses direct electric current to drive non-spontaneous chemical reactions. For this reason, aluminum requires an enormous amount of electricity. In fact, each U.S. aluminum factory uses an average of about 2,600 Megawatts (Streamline).
During the primary production process of aluminum, bauxite ore is imported from either Jamaica or South America (EIA). It is then converted into aluminum oxide by using natural gas as plants. After alumina is extracted from bauxite ore, smelting is used to convert the alumina into aluminum. Alumina is dissolved in a solution in which a strong electric current is applied. Since the smelting process requires a lot of electricity, some primary aluminum smelters can be found in areas with low-cost electricity—areas high in hydroelectric resources. Furthermore, during alumina refinement, some of the specific energy requirements include the energy sources of electricity, natural gas, oil and coal (Energetics INC, 30). All of these energy sources add up to a total of about 13,176 MJ (mega joules)/metric ton. With this number being so large, I’d assume that it is a quite energy-intensive.
Rather than work with bauxite, secondary production of aluminum uses aluminum scrap (EIA). Aluminum cans are mainly made from secondary aluminum since it is significantly less energy-intensive. In this secondary production process, aluminum-building materials are recycled. Furthermore, producing secondary aluminum requires cleaning and separating the aluminum scraps from other materials, then melting down the aluminum scraps in a furnace. The furnace is typically fired by natural gas, which is basically the energy source used in secondary production process. Evidently, the energy required for this secondary production is much less than primary production.
Semi-manufacturing and Manufacturing
When an aluminum ingot is produced, it can go through a variety of fabrication processes, such as rolling, extrusion, or casting, as a way to transform into different semi-products (Liu and Muller, 109). Typically, the aluminum ingots are continuously cast and fed into a rolling mill where they are rolled into plates, sheet or foil using a variety of hot and cold rolling processes (Energetics INC, 87). Cold rolling requires more energy than hot rolling because it takes more energy to roll the cold metal than it does a warm, flexible metal. The sheet then goes through selected finishing processes such as solution heat-treating, coating, marking, or packaging. When it comes to aluminum cans, coating is a popular finishing process. During the coating process, decorations are painted, sprayed, or rolled on at the mill. The major fuels used in casting and rolling are electricity and natural gas. Overall, I would assume that manufacturing and fabrication processes do require a variety of energy and sources, depending on which fabrication process is done.
Use
Once the aluminum has gone through production processes, it is ready to be used for transportation, building, packaging, consumer durables, and many more (Liu and Muller, 109). Aluminum has a big impact on building professions. Streamline mentions the building and construction industry is the second largest consumer of aluminum in the US, with the transportation industry being first.
While the building and construction industry is the second largest consumer of aluminum, aluminum is also known to be used for soda cans. There have been advancements in manufacturing technology, and therefore manufacturing technology has increased packaging efficiency, as well as sustainability, in the beverage industry (American Beverage Association). The packaging technology for the aluminum can industries include more than just how materials are used— it finds ways to make each step of the process in the aluminum can’s life cycle more efficient, from how the products are packaged to how much energy is used by the assembly lines. Since primary aluminum production is highly energy intensive, most aluminum can industries try to use the secondary production process as a way to reduce energy usage.
Waste management and Recycling
The rate at which old scraps are recycled usually varies depending on societal commitment and processing techniques available for different product categories.
According to the U.S. Environmental Protection Agency, recycling aluminum cans save 95% of the energy that would be required to make the same amount of aluminum from its source (American Beverage Association). In other words, aluminum only needs 5% of the amount of energy needed to create new aluminum when the metal is recycled. Unfortunately, only half of the aluminum cans that are consumed end up being recycled (Streamline). However, Americans aren’t very good at recycling in the first place.
While about 50% of aluminum cans are being recycled, Novelis, the largest American supplier of aluminum sheet, would like to raise that to 80 percent by the year 2020 (Wald). The reason for this goal is because of the amount of energy it takes to make virgin aluminum. As mentioned before, making an aluminum can from virgin aluminum requires enough fuel to make 3.5 kilowatt hours of electricity, but by recycling cans, the energy requirement would decrease to about one-eighth of that amount.
The Aluminum Beverage Can
So far, I’ve been researching the beginnings of the production of aluminum and the energy involved. While the previous information is relevant to the early life cycle of the aluminum can, I want to dive specifically into the process of producing the actual aluminum can.
The aluminum can production process begins with making the can sheet. This process begins with the conversion of ingots into can stock and lid stock coil which are then converted into aluminum can bodies and lids at the manufacturing plant (PE Americas, 69). Some time during the process, coils are annealed to give the aluminum the workability for downstream processing. Some manufacturing plants actually moved towards self-annealing, which requires no additional energy investment since the industry has improved their energy management.
The next step is shipping the aluminum coils to the actual can manufacturing plants. The coils go through a cupping press where blanks and discs are pressed into cups (PE Americas, 76). The cups then undergo forming, ironing, and punching operations to form the final profile of the can. An important task during can manufacturing is to ensure a flat top. To do this, the cans are trimmed at the top. After the trimming, the cans are washed and then dried.
Once the cans reach their final profile, the paints are applied externally for labeling and branding purposes (PE Americas, 76). They are also internally coated as a way to create a barrier between the metal and beverage. The aluminum beverage can is then necked, a process that reduces the diameter of the open end of the can to match the diameter of the lid. After this step, the flange that forms the seal to the lid is created. Furthermore, it should be mentioned that the lids of the can are manufactured by a different alloy than the body of the can. The alloys of lids have higher magnesium content as compared to the manganese used in the can body because the lids are designed to be stiffer than the body. While the cans may look the same, cans are grouped as either a two-piece or three-piece can; the difference is really just the amount of pieces used.
Now, after describing the processing of the actual can manufacturing, I want to emphasize the decorating and painting of the beverage can. When decorating three-piece cans, the decorations can either be printed on the can body or glued on if paper labels are used (EPA, 9). To decorate, inks are applied using the offset lithography process where inks are applied to a series of rollers that transfer the design onto the metal sheet. Then, an overvarnish is applied on top of the decoration while the inks are still wet. The ink and overvarnish have to be cured in a drying oven. The baking ovens have exhaust rates ranging from 1,500 to 8,000 scfm, which unfortunately, require a lot of energy (9). However, there are ultraviolet-radiation-cured (UV-cured) printing inks and UV overvarnishes that are cured by simply being exposed to ultraviolet radiation rather than heat, and therefore, the coatings don’t require passing through a drying oven. Two-piece cans go through slightly different decorating processes, but nevertheless must be dried in high production ovens. It is clear that printing on the cans require a good amount of energy but using UV coatings have the advantages of rapid curing and lower costs due to the elimination of drying ovens. The reasons only a few manufacturers use UV coatings are because the coating is more expensive and require special equipment.
After all the research, I can conclude that the life cycle of the aluminum can is very energy-intensive. The majority of the energy used comes from the production of aluminum itself. However, there are secondary production processes that require less energy. While there may be alternative ways to prevent so much energy usage, people must be willing to succumb to these options. A major step in preventing high-energy usage during aluminum can production is recycling. These little actions can really make a big difference in the environment.
Although I feel like I’ve learned a lot from all the research I’ve done, I feel that I failed to find enough information about the branding/decorating of the soda cans. Most of the information I encountered during my research of the life cycle of the aluminum can emphasized the production of aluminum. Nevertheless, I feel that the aluminum production is the most important part since it requires the most energy in the life cycle of the aluminum can. While most of my research didn’t really go in depth about the energy involved and required for making the can itself, I would assume that the printing and decoration of cans does require a good amount of energy—not just from drying the paints, but during the painting process. Furthermore, I came across little information about the energy embodied in lid manufacturing, though I’m sure there is plenty of energy that goes into that as well. Overall, I now understand that the production of aluminum cans isn’t simple at all, nor is it very eco-friendly. However, since people today are always looking to be more sustainable, I’m sure aluminum can manufacturers will succumb to more sustainable production options, such as the idea of paint-free soda cans.
Bibliography
American Beverage Association. "Sustainability." Packaging. American Beverage Association, 2013. Web. 12 Mar. 2013. <http://www.ameribev.org/minisites/recycling/packaging/sustainability.php>.
Bell, Judith. "The Life Cycle of Aluminum." Be Green Environmental Issues and Discussions The Life Cycle of Aluminum Comments. Best of the Web, 12 Jan. 2012. Web. 12 Mar. 2013. <http://begreen.botw.org /2012/01/the-life-cycle-of-aluminum/>.
EIA. "Energy Needed to Produce Aluminum." EIA. Energy Information Administration, 16 Aug. 2012. Web. 12 Mar. 2013. <http://www.eia.gov/todayinenergy/detail.cfm?id=7570>.
EPA. Control of VOC Emissions from Ink and Paint Manufacturing Processes. Environmental Protection Agency, 1992. Web. 12 Mar. 2013.
EPA. Preliminary Industry Characterization: Metal Can Manufacturing—Surface Coating. Environmental Protection Agency 1998. Web. 12 Mar. 2013.
Hosford, William F., and John L. Duncan. "The Aluminum Beverage Can." Scientific American (1994): 48-53. Web. 13 Mar. 2013.
Liu, Gang, and Daniel Muller. "Addressing Sustainability in the Aluminum Industry: A Critical Review of Life Cycle Assessments." Journal of Cleaner Production 35 (2012): 108-17. Web. 13 Mar. 2013.
Energetics INC. Energy and Environmental Profile of the U.S. Aluminum Industry. Publication. July, 1997. Web. 13 Mar. 2013.
Morrison, Sheila. "Life Cycle of an Aluminum Can." PRLOG. Press Release Distribution, 5 Oct. 2009. Web. 12 Mar. 2013. <http://www.prlog.org/10364317-life-cycle-of-an-aluminum-can.html>.
Parsons, Sarah. "Paint-Free Coke Can Saves Energy Paint-Free Coke Can Saves Energy, Reduces Pollution, Reduces Pollution." Inhabitat. Inhabitat, 23 Nov. 2009. Web. 12 Mar. 2013. <http://inhabitat.com /paint-free-coke-can-saves-energy-reduces-pollution/>.
PE Americas. Life Cycle Impact Assessment of Aluminum Beverage Cans. Publication. Boston: PE Americas, 21 May 2010. Web. 13 Mar. 2013.
Streamline. "Aluminum: How Sustainable Is It?" Streamline. Streamline, 2010. Web. 12 Mar. 2013. <http://www.streamlinemr.com/articles/aluminum-how-sustainable-is-it>.
The Aluminum Association. "News & Statistics." Aluminum. The Aluminum Association, 2008. Web. 12 Mar. 2013. <http://www.aluminum.org/AM/Template.cfm?Section=News_Statistics>.
UNEP. Why Take a Life Cycle Approach? United Nations Publication, 2004. UNEP. Publication. Web. 13 Mar. 2013.
Wald, Matthew L. "Toward a Greener Soda Can." New York Times. New York Times, 12 June 2012. Web. 12 Mar. 2013. <http://green.blogs.nytimes.com/2012/06/12/toward-a-greener-soda-can/>.
Debra Cheung
Professor Cogdell
DES40A
13 March 2013
Aluminum Beverage Cans: Waste and Emissions from Logo Printing
Just for a dollar, a little kid can purchase a Coca-Cola soda can from the convenience store. The bright red luster color with the script logo is the main feature that attracts consumers every day. However, they buy more than just the liquid content inside of the can: they buy the chemicals that went into the production of aluminum, the environmental damage from manufacturing the body and the lid, and the emissions released into the air. Despite the variety of wastes generated from the production of soda cans, aluminum has many inherent properties that make it better to use than other metals like steel and iron. Although aluminum soda cans have an impressive life cycle (as they are upcyclable), it remains a challenge to discover more sustainable solutions for the wastes and emissions generated from the entire process.
Charles Hall was the first to extract pure aluminum through electrolysis, and since then aluminum has made a tremendous impact in all industries, from beverage cans to cookware. Aluminum is lightweight and durable, and it "provide the strength of steel at only a third to half the weight" (“Aluminum: The Element of Sustainability” 4). Aluminum also boasts the quality of being upcyclable, meaning that it does not end up in landfills, but instead it is reused continuously for other products. This makes aluminum a very promising metal because then it is possible to develop energy efficient products that give back to the environment, yet provide the same quality service to customers. Certain manufacturers achieve this life-cycle way approach towards aluminum (and other materials) more successful than others do. The excess wastes from aluminum smelting and the production of beverage cans represent an area that designers and the business industry can join together to figure out how to minimize waste, but maintain the integrity of the product. Millions of aluminum soda cans are annually bought and often its use is just about five minutes; its manufacturing starts in extracting bauxite or recycled scrap (“Aluminum: The Element of Sustainability” 20).
Aluminum is derived from the extraction of bauxite, which can be found in mining ores in certain deposits. Most bauxite is imported to the U.S. from deposit ores such as Jamaica and Guinea (Woodward). The extraction of bauxite has a huge toll on the environment because it "requires tree and vegetation removal; habitat disturbance water, land and energy use; and generates solid wastes" (“Aluminum: The Element of Sustainability” 20). Not even the production of the actual soda can has begun, and already a significant amount of waste is generated just by extracting the bauxite. Pollutants from the solid wastes spread to the air and in the water as well. These pollutants heavily disrupt the lives of bird species and aquatic animals, which results in death and mutation. After bauxite is extracted, it then continues with the next phase in aluminum smelting: alumina refining.
Alumina refining is extracted from the bauxite by the Bayer process. The Bayer process ultimately causes the bauxite to go through several phases, including crushing and digesting. The waste that results from the Bayer process is red mud. Red mud is a reddish colored sludge fill that mainly is comprised of "dissolved aluminum oxides (sodium aluminate, NaA1O2) and insoluble bauxite resides" (Margolis 27). Disposing red mud is problematic because it contains hazardous materials unfit for the environment. To deal with this issue, red mud generally is placed into specific locations "in the form of lagooning/ponding, dry stacking, or dry cake" (“Aluminum: The Element of Sustainability” 23). It is intriguing to note the potential ways of safely disposing (or using) red mud due to its unique combination of minerals. Perhaps in the future red mud might have a sustainable use, but currently there are no better solutions for it. Solid wastes like red mud are unavoidable because they are leftover residues, so any solution would have to be extraordinary to prevent it from forming or to minimize it.
There are two different ways of manufacturing aluminum: "primary production and secondary production" (“Aluminum: The Element of Sustainability” 24). There is a comprehensive amount of information on these two specific ways of manufacturing aluminum and the wastes that are produced, which was definitely a success on my part. Both methods are very extensive, but in general primary production involves smelting and secondary production involves using scraps as the material. Within the primary production, there are two ways of smelting: "the Soderberg technology and the pre-baked technology" (“Aluminum: The Element of Sustainability” 24). The later technology is much older, which requires more energy use and emits more emissions. Because primary production requires a huge amount of electricity and heat, airborne wastes are born. Some of these airborne wastes include "greenhouse gas emissions [and] fluoride emissions" (“Aluminum: The Element of Sustainability” 25). Both of these pollutants are toxic, especially fluoride. The EPA mandates that the level of fluoride production be below a certain level in aluminum production factories because it is a toxic gas.
Scraps are the main material in secondary aluminum production. This is what makes aluminum a propitious metal: the ability to use scraps of aluminum metals and other alloys to create more metal that is "new" but made out of "old” material. Scraps are sorted whether they are usable or not and then are melted. From secondary aluminum production, air emissions are expelled, such as "chloride gases, volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons" (“Aluminum: The Element of Sustainability” 28). It is difficult to not have any wastes as a result from these processes because by using heat and electricity, there will always be some type of waste, whether it is solid wastes or air emissions. However, laws have been created to ensure that the aluminum industries cut down its harmful impact to the environment, and they actually strive to be as clean as possible in order to reuse certain solid wastes like "salt cake" and to control air emissions. It surprises me how much aluminum goes through before even going into production of the items that we use daily, such as foil and soda cans.
Carbon dioxide is particularly high in number from the production of aluminum. Although the number of fluoride emissions has dramatically decreased, carbon dioxide emissions are high because it "is the main component of the gas created during electrolysis of alumina to form aluminum" (Margolis 22). They account for more than half of the total air emissions in the smelting process. This is an incredibly significant percentage, and the percentage comes from the fact that carbon dioxide depends on electric power. There is a possibility that the number of carbon dioxide emissions will decrease if the electric power source switches "from coal fired to hydroelectric power generation" (Margolis 23). Aluminum smelting outputs considerable amounts of waste and emissions that erode habitats and worsen the air quality. The production of the beverage can itself also proves to disperse its own unique wastes.
The final product of aluminum is sent out to beverage can industries. As stated early, beverage soda cans are two-piece bodies that comprises of the body and the lid. They are made usually out of the method called the "two-piece drawing and wall ironing" started by Reynolds Metals (Hosford and Duncan 50). The first step is cutting them out of circular blanks (which are sheets of aluminum cut into a circle). Obviously, the byproduct of this step is the leftover scraps from cutting the circles out. Manufactures make the sheets "wide enough to incorporate 14 cups laid out in two staggered rows" to avoid the immense scrap loss (Hosford and Duncan 50). At least the scraps would not be a total waste as they serve as the main material for secondary aluminum production. This exemplifies the salient qualities of aluminum because the scraps are not considered waste, but instead represent a constructive way of material reuse.
After the cans are punched out from the blanks, they need to be redrawn to the exact size. This step involves the drawing and ironing– the machine cuts the cans into a more accurate diameter, which increases the height, thins the cup walls, and stretches out the cups with the bottom bulging out. At this stage, the cans are now starting to look more like the average soda cans. The small ripples at the top need to be trimmed, and they occur because it "is an unavoidable effect of the crystalline structure of the aluminum sheet" (Woodward). That provides another source of scraps that is fitting for secondary aluminum production. The lids of the cans are actually made out of a different alloy "with more magnesium and less manganese" than the body because they has to be stronger than the base to handle the internal pressure of the can (Woodward). They are cut out as well, which produces more scraps for secondary aluminum production. After the cans have been cleaned, sprayed, and decorated, the necks are formed and then the cans are filled with a liquid of the beverage.
Beverage soda cans endure through several washings in the cleaning stage. It undergoes "a pre-wash, a wash, a rinse, a treatment stage, a city-water rinse, and a deionized water rinse" (Kirsch and Looby 2). A substantial amount of water waste is born from this step. It involves "solutions of either sulfuric, hydrochloric, or hydrofluoric acid to etch the can surface to promote ink/overvarnish adhesion" (“Preliminary Industry Characterization” 28). This is the major source of wastewater from can manufacturing companies, and this is unfortunate because it makes the waters unsuitable for marine animals to thrive and it also takes water away from the city. Even the machines and the work practices play a role in either reducing or increasing emissions. Air emissions from the cleaning process appear to be low as "less than 1 ton per year and are typically uncontrolled" (“Preliminary Industry Characterization” 28). However, it is demanding to fabricate methods to avoid air emissions completely, so instead methods are often devised to create the lowest impact as possible. A variety of ways are available to dispose wastewater: they can be transferred to tanks where they are broken down, cleaned to be sent to another companies, or disposed through the sewer (which is extremely hazardous).
The painting, coating, and inking process emit hazardous air pollutants (HAP). The process of how the paints and inks are made is important because depending on how they were made, it contributes to the overall emissions of the aluminum can process. One type of coating is solvent borne coating, which actually has "high concentrations of VOC's, typically 4.0 to 6.6 pounds of VOC per gallon of coating" (“Preliminary Industry Characterization” 19). This was the conventional way to coat cans because it had certain advantages to other methods of coating, such as "good abrasion resistance... and easy application" (“Preliminary Industry Characterization” 19). Solventborne coating also use can inks that are alkyd-based and that do not have HAP. They no longer are coated on beverage cans because of the intense presence of VOCs.
High-solid coatings and waterborne coatings are two other types of coating methods applied to beverage cans. High-solid coatings have a lower content in VOCs and can be used in either low-process can manufacturing or in high-process can manufacturing. The inks that are involved in beverage can manufacturing "are polyester-based" and solid, and therefore the method of printing on cans with solid inks is called dry offset lithography (“Preliminary Industry Characterization” 20). Waterborne coatings are frequently used because water is the main material and it has organic solvents; this produces fewer concentrations of VOCs. The trend of using waterborne coatings proves that sustainability is an important issue and that other manufacturing companies might catch on. Depending on the coating method, they typically are applied to the interior and exterior sheets to give the polished look and the strength capable of holding the liquid without damaging it from the chemicals used in the coating process. An assortment of air pollutants and chemicals results from the coating process, including certain glycol ethers and methanol. The emissions that are released during the coating process of the can depends on the method of coating and the raw materials that are used within the inks and paints themselves.
Once the cans have been distributed and used, most people just recycle their cans (unless they are lazy and dump them somewhere else). Recycling is considered such an easy way to lessen the hazardous impact on the environment, but it proves ironically difficult in "developed, advanced economies" because it might be considered "too easy" that people do not do it "(“Aluminum: The Element of Sustainability” 53). My speculation on this problem is that since people constantly see soda cans in stores, they might think it is okay to recycle the next can they drink because there will always be another soda can to recycle. Aluminum is such a sustainable material that it would be unfortunate and a huge loss if each piece ended up in the landfill. Aluminum beverage cans are sorted at material recovery facilities (MRF) and then are delivered to industries that sell scrapped metals. During the recycling process, can flatterers are used to process the cans. It is probable that the can flatterers emit some sort of emissions because they process the metals and the leftover aerosols from post-consumed aerosol containers. I could not find exact information on the wastes from the recycling process as I kept finding information on how to recycle. This presented a similar problem in finding the exact manufacturing processes of aluminum soda cans for specific companies.
Companies like Coca-Cola have adopted Metals' way of manufacturing aluminum soda cans. Though I could not specifically find the manufacturing process of Coca-Cola (because they might not want to expose their work practices or their factories), I would safely assume that Coca-Cola still practices the two-piece drawing and wall ironing because their soda cans symbolize the standard aluminum can. Information regarding the specific process of logo branding of cans was difficult to find as I ended up with sources that talked about different soda brands and advertisements about beverage can printing companies. My group members also suffered the same problem. Otherwise, information about aluminum smelting, the wastes and hazards of each steps, and the production of beverage soda cans is very plentiful. It amazes me how each step of the aluminum can has its own complexities that most people are not aware of it.
Although each step of aluminum can making creates waste, its overall use overlooks that fact. Aluminum is extremely valuable, and there are even markets that exist based on scrapped metals. I had no trouble finding information on aluminum and where it was derived and how it was made because it appears as general knowledge. However, when it came down to specifically what machines, what work practices, where they receive their materials from, and what logo branding techniques, those areas of information seem secretive. Aluminum production is not entirely eco-effective since problems of solid wastes and air emissions continue to persist; however, that does not stop aluminum from being widely used in all sorts of appliances and items and perhaps inevitably reducing global emissions.
Bibliography
"ACLCA - Learn." ACLCA - Learn. Web. 28 Feb. 2013. <http://www.lcacenter.org/learn.aspx>.
"Alcoa RPD: About Alcoa Rigid Packaging: How Aluminum Cans Are Made." Alcoa RPD: About Alcoa Rigid Packaging: How Aluminum Cans Are Made. Web. 7 Mar. 2013. <http://www.alcoa.com/rigid_packaging/en/about/making_cans.asp>.
Aluminum: The Element of Sustainability. Rep. The Aluminum Association, Sept. 2011. Web. 7 Mar. 2013. <http://aluminum.org/Content/ContentFolders/Miscellaneous/Aluminum_The_Element_of_Sustainability.pdf>.
Aneziris, O. N., I. A. Papazoglou, and O. Doudakmani. "Assessment of Occupational Risks in an Aluminium Processing Industry." International Journal of Industrial Ergonomics 40.3 (2010): 321-9. ProQuest. Web. 13 Mar. 2013.
"Beverage Cans: Overview." Can Manufacturers Institute. Web. 7 Mar. 2013. <http://www.cancentral.com/bevCans.cfm>.
Emission Estimation Technique Manual for Aluminium Smelting. Rep. Emission Estimation Technique Manual for Aluminium Smelting. National Pollutant Inventory, Mar. 1999. Web. 24 Feb. 2013. <http://www.npi.gov.au/publications/emission-estimation-technique/pubs/falsmelt.pdf>.
Hershenson, Roberta. "Finding Out what Happens to all that Recycled Material." New York Times: A.1. ProQuest. Oct 31 1993. Web. 13 Mar. 2013 .
Hosford, William F., and John L. Duncan. "The Aluminum Beverage Can." Chymist.Com. Sept. 1994. Web. 18 Feb. 2013. <http://www.chymist.com/Aluminum%20can.pdf>.
"Journals." Australian Life Cycle Assessment Society. Web. 28 Feb. 2013. <http://www.alcas.asn.au/resources/journals>.
Kirsch, F. William, and Gwen P. Looby. Environmental Research Brief. National Service Center for Environmental Publications (NSCEP). Web. 24 Feb. 2013. <http://nepis.epa.gov/Exe/ZyNET.exe/300024M1.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3AzyfilesIndex%20Data91thru94Txt0000001300024M1.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h|-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p|f&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL>.
Margolis, Nancy. Energy and Environmental Profile of the U.S. Aluminum Industry. Rep. U.S. Department of Energy, July 1997. Web. 24 Feb. 2013. <https://www1.eere.energy.gov/manufacturing/resources/aluminum/pdfs/aluminum.pdf>.
Markets for Recovered Aluminum. Rep. United States Environmental Protection Agency, Apr. 1993. Web. 8 Mar. 2013. <http://www.epa.gov/wastes/nonhaz/municipal/pubs/sw90077a.pdf>.
McMinn, B.W., and P.J. Marsosudiro. Control of VOC Emissions from Ink and Paint Manufacturing Processes. Rep. United States Environmental Protection Agency, Apr. 1992. Web. 8 Mar. 2013. <http://www.epa.gov/ttn/catc/dir1/ink_paint.pdf>.
Preliminary Industry Characterization: Metal Can Manufacturing--Surface Coating. Publication. U.S. Environmental Protection Agency, Sept. 1998. Web. 6 Mar. 2013. <http://www.epa.gov/ttnatw01/coat/mcan/pic-can.pdf>.
Smith, D. N., et al. "The use of can Flatteners in MRFs for Processing Material Including Post Consumer Aerosols - a Risk Assessment." Resources, Conservation and Recycling 31.2 (2001): 115-35. ProQuest. Web. 13 Mar. 2013.
"Why Take a Life Cycle Approach." UNEP DTIE SCP Branch: Publications. United Nations Environment Programme, 2004. Web. 28 Feb. 2013. <http://www.unep.fr/scp/publications/details.asp?id=DTI/0585/PA>.
Woodward, Angela. "How Products Are Made." How Aluminum Beverage Can Is Made. Made How. Web. 24 Feb. 2013. <http://www.madehow.com/Volume-2/Aluminum-Beverage-Can.html>.