Design Life-Cycle
assess.design.(don't)consume
Claire Zhao
Professor Cogdell
DES40
15 March, 2018
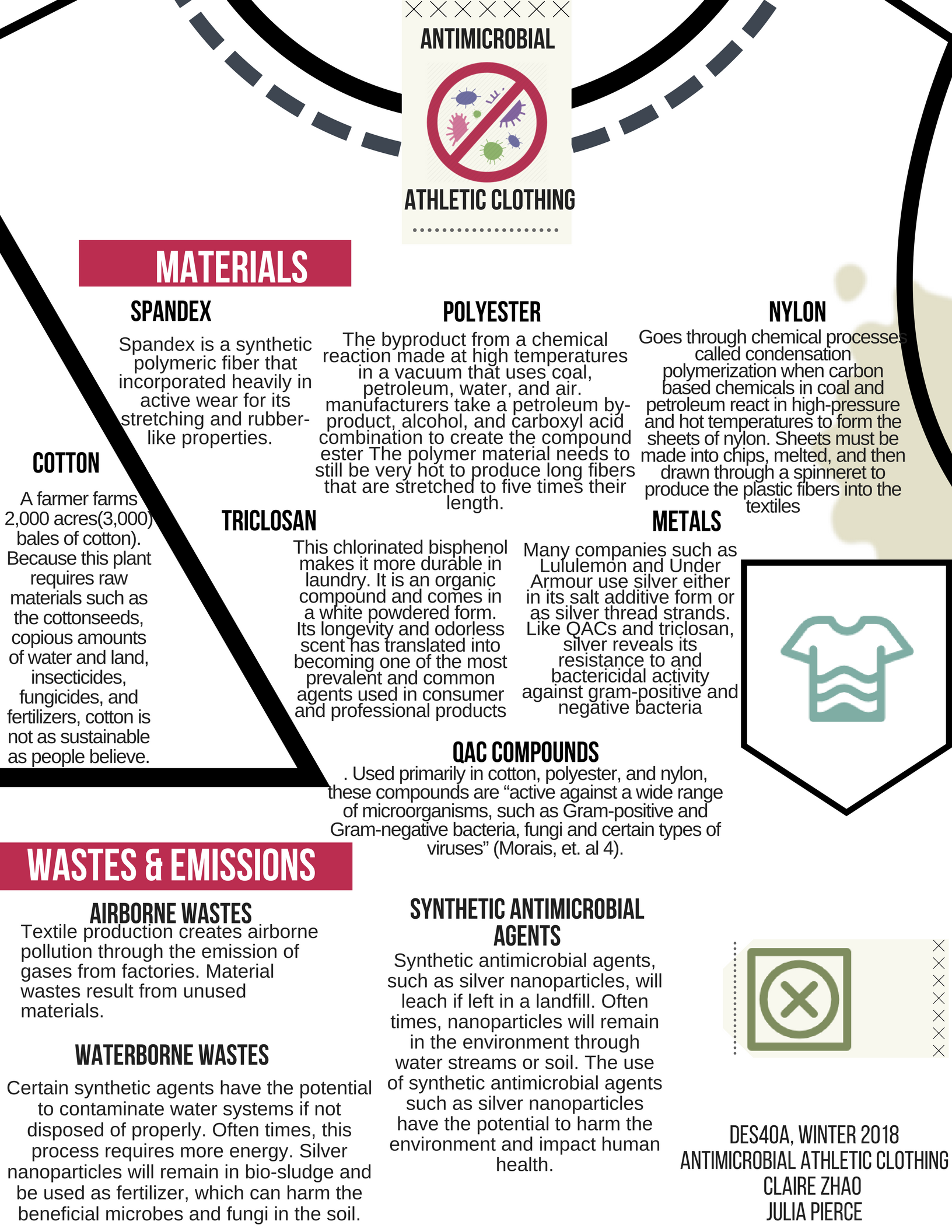
Materials in Anti-Microbial Athletic Clothing
For this DES40 project, our group was initially interested in researching Lululemon’s leggings because of the recent success and popularity in the brand among young adults. From browsing the Lululemon website, we discovered one of their lines called Silverescent, products that boast of their antimicrobial properties through the use of silver threads. We became increasingly interested in how textiles can utilize silver threads and other antibacterial materials to kill and prevent the spread of bacteria. While modern technology has implemented materials such as silver, triclosan, ammonium compounds, etc, that kill and inhibit the growth of bacteria left on athletic wear, case studies suggest that while there are various manufacturing processes, the general efficacy of such clothing is negligible and harms the environment due to its poor disposability.
Antimicrobials focus on killing and inhibiting the growth of bacteria and fungi that more specifically flourish on human skin. Thus, antimicrobial textiles have antimicrobial agents integrated into or onto the fibers’ surfaces to inhibit the growth of harmful microorganisms. [1] Companies like Dow Microbial Control, Microban, Lululemon, Under Armour, etc, manufacture products that guarantee sweat stain free and bacteria killing technology. Lululemon uses X-Static technology, which embeds 99.9% pure silver in its fibers. [2] Companies rely on the chemical energy and reactions in their materials to deliver their antimicrobial qualities.
The most common fibers in textiles used to make clothing are denim, polyester, spandex, and cotton, which accounts for about 33% of all the textiles made (Drew & Yehounme). Cotton, spandex, and lycra make up a majority in the composition of athletic clothing and are designed to “wick” sweat away while also being breathable. Athletic gear is also made of synthetic and plastic based fibers that keep moisture on the surface of garments to dry more quickly (Dion). When brands promise antimicrobial functionalities, the most common antimicrobial compounds include triclosan, metals such as silver, polybiguanides, and natural compounds with long alkyl chains. Researchers Morais, Guedes, and Lopes examine that the antimicrobial action of compounds depend on their alkyl chain lengths, the presence of the perfluorinated group and the cationic ammonium group’s number in the molecules” (Morais, et. al 3). The longer the alkyl chain, the more water repellency the agent has. This is why the most commonly used materials in athletic wear are cotton, polyester, and nylon because of their long alkyl chains and ability to wick moisture.
Spandex is a synthetic polymeric fiber that incorporated heavily in active wear for its stretching and rubber-like properties. It is made up of polyester and polyether polyols chains that are known for their rigidity and tensile qualities, allowing spandex to stretch to 600% its size. [3] The chains of polymers, polyurethane, is made into a fiber by using a dry spinning technique where a solution of fiber forming material and a solvent is used with hot air through a spinneret. Spandex, much like water, has a unique and random molecular structure because the fibers compose of two kinds of segments, long amorphous segments and short rigid segments. [4] Having a combination of free form and rigid strands is what gives spandex its durability and resistance to breaking. Spandex is typically blended with materials such as cotton and wool to offer a lightweight feel and it also resists moisture and body perspiration well.
Polyester is another material used in active wear and is similar to spandex because of its long chain of polymers but with properties closer to plastic than to rubber. Its long chain of polymers is called polyethylene terephthalates that is created by a combination of glycol and terephthalic acids. [5] Because polyester fibers are quite durable, they are also resistant to stretching, which makes it a good material for active wear. Synthetic polyester is the byproduct from a chemical reaction made at high temperatures in a vacuum that uses coal, petroleum, water, and air. [6] Through a reaction called polymerization, manufacturers take a petroleum by-product, alcohol, and carboxyl acid combination to create the compound ester that is part of polyester. From there, the polymer material needs to still be very hot to produce long fibers that are stretched to five times their length. Like spandex, polyester undergoes spinning processes: filament or spun. In the filament spinning type, the fibers are twisted together, while the spun type have short pieces of the fiber to form a “staple”. These methods make it more convenient for polyester to be blended with cotton or wool, other common materials used in athletic gear. Because polyester is made from petroleum, a crude oil, this is a non-renewable and non-biodegradable source that utilizes a large amount of heat and power to produce. While it does not biodegrade, it can be recycled into another form of plastic. [7]
Nylon is another synthetic and plastic-like material used in active wear that comes from crude oil. Nylon also goes through chemical processes called condensation polymerization to form a large polymer that thus creates the stretchy and resilient fibers popular in athletic clothing. Condensation polymerization is when carbon based chemicals in coal and petroleum react in high-pressure and hot temperatures to form the sheets of nylon. To integrate nylon into fabric, the sheets must be made into chips, melted, and then drawn through a spinneret to produce the plastic fibers into the textiles. [8]
While nylon, spandex, and polyester are useful for their stretchy and resistant to breakage, they are all synthetic materials made from chemical byproducts of crude oils. These materials indirectly impart on the environment greenhouse gases, water shortages, and high energy use. “Producing nylon creates a gas called nitrous oxide that is 310 times more harmful than carbon dioxide” (Uren). Furthermore, spinning and cooling these fibers require large amounts of water and energy that also deplete the environment’s natural resources. 9
Aside from synthetic fibers, cotton is a primary material used in athletic gear because of its comfort and versatility. Cotton comes from a plant in the Mallow family with sticky seeds that must be separated from its wool in order to continue the spinning and weaving processes. Once the cotton is de-seeded, it is cleaned, fibers aligned, spun into yarn, and then woven into fabrics. Growing cotton, however, is costly and can be just as environmentally inefficient as manufacturing synthetic textiles. For cotton to be economically viable, a farmer must farm about 2,000 acres, which produces about 3,000 bales of cotton. [9] Because this plant requires raw materials such as the cottonseeds, copious amounts of water and land, insecticides, fungicides, and fertilizers, cotton is not as sustainable as people believe.
Depending on the manufacturer and budget, textiles can be made antimicrobial in a variety of ways: “as a finish by exhaust, pad-dry-cure, coating, spray, and foam techniques, or the substances can be applied by directly adding into the fiber spinning dope” (Hoefer and Hammer 2). Whatever method is chosen, however, the antimicrobial compounds are always integrated in the clothing’s finishing stages on the material’s surface. Antimicrobial agents work through contact, which means the antimicrobial agent does not disperse and keeps the microorganism in one spot, and/or through diffusion, where the antimicrobial agent is infused in the fiber surface allocates itself from the textile to the “external medium to attack the microorganism” (Morais, et. al 3).
The most common antimicrobial compounds used in textiles are triclosan, QAC compounds, metals such as silver, and chitosans. QAC compounds stand for Quaternary ammonium cation compounds and are used heavily in hygienic and cleaning products due to the presence of their long alkyl chains (12-18 carbon atoms). Used primarily in cotton, polyester, and nylon, these compounds are “active against a wide range of microorganisms, such as Gram-positive and Gram-negative bacteria, fungi and certain types of viruses” (Morais, et. al 4). In Morais’ study, she and her other team members concluded that QACs in textiles demonstrate their antibacterial properties based on how surface charges and cell membrane charges interact. With a positive surface and a negative cell membrane charge, this results in the bacteria losing its “membrane permeability and cell leakage”. However, while QACs demonstrate a high efficiency against Gram-positive and negative bacteria and other fungi, these compounds leach from textiles since they are not bonded with it and decrease the lifespan and reusability of the textiles. From a sustainability standpoint, clothing made with QACs is not the most effective since they lose almost all of its antimicrobial properties and can be “directly exhausted under near boiling conditions when washed” (Morais, et. al 4).
While QAC compounds do not perform well under long periods of time, triclosan is a material used in making antimicrobial clothing that is more durable and more prevalent in the textile and hygiene industries. Because it is not ionized and because of its synthetic makeup, this chlorinated bisphenol makes it more durable in laundry. It is an organic compound and comes in a white powdered form. Its longevity and odorless scent has translated into becoming one of the most prevalent and common agents used in consumer and professional products such as soaps, lotions, toothpastes, mouthwashes, etc, over the last thirty years. Triclosan is also mainly used “in association with polyester, nylon, polypropylene, cellulose acetate and acrylic fibers” (Morais, et. al 5). However, using triclosan has its own consequences, especially when incorporated in clothing. Because triclosan has only ever been used for hygiene, implementing this agent into textiles for its antimicrobial effects has become a concern not only because of its toxicity because of its “potential for selective pressure for certain antibiotic cross-resistant strains, generating bacterial resistance”, but also because of the toxicity of the “photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solutions ” (Morais, et. al 5).
Another agent incorporated in athletic wear is metal, more specifically silver, for their antibactericidal qualities and its greater ability to bond to other compounds. Many companies such as Lululemon and Under Armour use silver either in its salt additive form or as silver thread strands. Like QACs and triclosan, silver reveals its resistance to and bactericidal activity against gram-positive and negative bacteria. When the silver reacts with moisture, its ions are released and will damage and inhibit any bacterial RNA and DNA replication in proximity, namely strains such as “aeruginosa, s. aureus, staphylococcus epidermis, e. coli and klebsiella” (Morais, et. al 5). However, similar to triclosan, bacteria can also develop resistance to such agents like silver, thereby diminishing textiles’ antimicrobial performance. In addition, with prolonged wear and washes, the silver may even release odor.
No matter the antimicrobial agent or method implemented into the textiles, they all share common themes such as the concern for their byproduct waste management and poor disposability. Antimicrobial clothing is a highly profitable industry that in 2000 “was estimated that the production of antimicrobial textiles reached about 30,000 tonnes in Western Europe and 100,000 tonnes worldwide. Moreover, between 2001 and 2005, in Western Europe it was reported an annual production increase of antimicrobial textiles around 15%, being one of the fastest growing sectors of the textiles industry” (Morais, et. al 2). These numbers demonstrate the profitability of antimicrobial clothing but also its overconsumption. Over 100,000 tons of these textiles are produced but also then recycled or disposed of improperly. “silver, triclosan, trichlorocarban, and other germ and odor-killing compounds wash away very quickly, some just after three washings while others after ten,” (Zerbe). Furthermore, not only are these antimicrobial agents variable in their functions, such compounds are also very toxic to the human body and to the environment. However, Researcher Robert Reed and his team resolve that using antimicrobial agents, such as silver (Ag) in moderation is possible. In their research, by lowering the amount of silver and tethering the silver nanoparticles to the textiles, they were able to maintain high antimicrobial efficacy while reducing the amount of leaching. If manufacturers use very small amounts of silver, say silver loadings as low as a few parts per million (μg/g), the silver will not wash out” (Reed, et. al 9). In support, researcher Paul K. Westeroff and his colleagues suggest lowering the amount of antimicrobial agents in textiles in order to reduce their environmental impact while also being able to prevent microbes from becoming too resistant (Environmental Science & Technology).
Antimicrobial clothing does not need to rely on synthetic and chemical compounds such as silver, triclosan, alkyl chain compounds that end up in waste and landfills and increase the amount of toxins in the environment. The KTH Royal Institute of Technology writes of a colleague’s discovery of new antibacterial fibers that combine “bio-compatible plastics with the antimicrobial compound, lanosol, which is commonly found in seaweeds of the family Rhodophyta, or red algae” (KTH Royal Institute of Technology). Using a process called electro-spinning, they have been able to create a thin thread made of red algae, which is a more safe and ecologically friendly alternative to other antimicrobial agents such as triclosan and metals.
Works Cited
[1] http://www.apparelsearch.com/terms/a/antimicrobial-fabric-term.html
[2] https://info.lululemon.com/design/fabrics-technology/silverescent
[3] https://pubs.acs.org/cen/whatstuff/stuff/7707scitek4.html
[4] http://www.madehow.com/Volume-4/Spandex.html
[5] http://www.whatispolyester.com/
[6] http://www.craftechind.com/how-is-polyester-made/
[7] https://www.trustedclothes.com/blog/2016/04/21/ethical-fabrics-to-consider-the-ugly-draft/
[8] https://goodonyou.eco/material-guide-nylon/
[9] http://www.madehow.com/Volume-6/Cotton.html
Bibliography
Dion, Gabrielle. “Comparison of Fabric for Exercise Clothing.” Live Strong. 11 September 2017. https://www.livestrong.com/article/414298-comparison-of-fabric-for-exercise-clothing.
Drew, Deborah, and Genevieve Yehounme. “The Apparel Industry's Environmental Impact in 6 Graphics.” World's Resources Institute, World's Resources Institute, 5 July 2017, www.wri.org/blog/2017/07/apparel-industrys-environmental-impact-6-graphics.
Environmental Science & Technology. “The Impact of Anti-Odor Clothing on the Environment.” ACS, American Chemical Society, 30 Mar. 2016, www.acs.org/content/acs/en/pressroom/presspacs/2016/acs-presspac-march-30-2016/the-impact-of-anti-odor-clothing-on-the-environment.html.
Hoefer, Dirk, and Timo R. Hammer. “Antimicrobial Active Clothes Display No Adverse Effects on the Ecological Balance of the Healthy Human Skin Microflora.” ISRN Dermatology 2011 (2011): 369603. PMC. Web. 4 Feb. 2018.
KTH Royal Institute of Technology. “Safer Than Silver: Antibacterial Material Made With Algae.” Phys.org, Science X , 1 Oct. 2014, phys.org/news/2014-10-safer-silver-antibacterial-material-algae.html.
Morais, Diana, et al. “Antimicrobial Approaches for Textiles: From Research to Market.”Materials, vol. 9, no. 12, 2016, pp. 1–21., doi:10.3390/ma9060498.
Reed, Robert B., et al. “Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles.” Environmental Science & Technology, vol. 50, no. 7, 29 Feb. 2016, pp. 4018–4026., doi:10.1021/acs.est.5b06043.
The BioCote Team. “The Use of Antimicrobial Textiles.” BioCote, BioCote, 15 June 2016, www.biocote.com/blog/use-antimicrobial-textiles/.
Thomas, Pat. “Antibacterial Clothing - A Fashionable Threat to Human Health.” Natural Health News , Natural Health News , 29 Dec. 2011, www.naturalhealthnews.uk/environment/2011/12/antibacterial-clothing-a-fashionable-threat-to-human-health/.
Uren, Ashlee. “Material Guide: How Sustainable Is Cotton?” Good On You, Good On You , 22 June 2016, goodonyou.eco/material-guide-nylon/.
Zerbe , Leah. “Germ-Resistant Clothes: Pick or Pass?” ABC News, ABC, 11 Mar. 2012, abcnews.go.com/Health/Wellness/germ-resistant-clothes-pick-pass/story?id=15885681.
Julia Pierce
Professor Christina Cogdell
DES 40A Section A02
13 March 2018
Wastes & Emissions of Antimicrobial Clothing
Products that incorporate antimicrobial agents have widespread demand in many facilities and homes in order to guarantee a barrier between an individual and bacteria. Antimicrobial agents are used within environments that require high levels of sterilization such as medical facilities, factories, and more. Products include disinfectants and sanitizers, but more recently, fibers. Antimicrobial textiles have become widely used and marketed in order to distribute a product that fights bacterial growth in clothing. However, the spread of antimicrobial clothing has the potential to have negative impacts. There is little research on the effects of the wastes and emissions of antimicrobial textiles, but the information that does exist reveals a large amount of particles that remain in the environment. Antimicrobial clothing is an important advancement in limiting bacterial growth in textiles through nanoparticles; however, the large impact of its wastes and emissions harms the environment and the people within it.
Antimicrobial textiles are defined as fibers that have had antimicrobial agents applied to their surface in order to kill or inhibit the growth of any bacterial substance1. While antimicrobial fibers have had a long use in medical facilities such as hospitals, they are becoming more popular to consumers. Over time, people have become more aware of the threat of bacterial infections and the action to prevent infection has become more common. Because fabrics have the ability to retain moisture, microorganisms can easily grow2. This is especially prominent in workout gear, where sweat and increased temperatures can lead to the spread of bacteria and fungi. As a result, a large market has been built around antimicrobial clothing in exercise wear. The goals of antimicrobial textiles may seem like a necessary solution to bacterial growth, however the process of creating these products is not always ecologically friendly. There are some environmentally sound and natural approaches to applying antimicrobial agents to fibers, however other applicants produce negative wastes and emissions that go back into the environment. These wastes and emissions can be transmitted through water, air, or through any product that returns into a cycle in the atmosphere. Therefore, while there are benefits from the bacterial resistance that antimicrobial textiles create, more attention needs to be placed on which agents are being used and the methods used to process them.
The antimicrobial result is obtained through the process of applying the agent to the fibers3. This can be done through either physical or chemical processes. In a physical process, textiles become antimicrobial through the spinning or extrusion process, while additives are introduced4. However, the textile industry produces a large amount of waterborne and airborne wastes in addition to solid wastes and odors5. These wastes are classified by either dangerous or non-dangerous wastes. Wastes that are dangerous include chemicals, dyes, and electronic waste, while non-dangerous wastes are made up of the textiles themselves (such as yarn, fabrics, and fibers) and the materials needed to package them (such as cardboard and paper)5. While physical practices can be more environmentally sound than chemical processes, the methods in which the textiles are produced still produce a large amount of wastes. Alternatively, in a chemical process, cationic polymers can also be added, which alter the structure of cell membranes6. By changing the cell’s function or integrity, the antimicrobial agents have the ability to kill the microorganism2. However, this introduces an issue with emissions of antimicrobial agents into the environment. Although the chemical additives are effective, they are more susceptible to leaching when washed due to a lack of physical bonding2.
Because of its properties and ability to kill bacteria, silver nanoparticles are a popular antimicrobial agent2. Silver nanoparticles, also called AgNPs, have become widely used, but little information is known about their effects on human health and the environment. However, published research reveals the detrimental effects on silver against cells, and therefore warns against its excessive use7. Research on AgNP concludes that it degrades cytoskeleton components, upsets pre and post-synaptic proteins, and causes mitochondrial dysfunction, all of which lead to cell death7. However, AgNPs are still being used. In addition, another experiment tested antimicrobial efficacy to impact of silver in multiple life stages, from being washed to being left in a landfill. Results revealed that while washing the antimicrobial textiles did release silver, it did not affect the efficacy; this shows that only a small amount of AgNP is needed to control microorganism growth.8 Therefore, overusing the amount of silver needed in the fibers can be considered material waste. In addition to solid waste, silver will also leach from textiles while in a landfill7.
Therefore, when considering the benefits versus the harmful effects of the wastes and emissions of antimicrobial textiles, it is important to consider which agent is being applied. There are more natural agents, which can come from plant or herb-based compounds2. There is little research about these agents and how they work; however, it is suggested that their multiple action mechanisms work against resistant bacteria strains2. Other possible natural agents include Chitosan, which is made up of Chitin, a substance extracted from the exoskeleton of crustaceans, and other natural amino acids and proteins2. When considering wastes, Chitosan is derived from waste shrimp and crustacean shells9. However, all of these natural polymers are dependent on molecular weight, pH, and temperature2,9. This makes it difficult to rely on these agents and in turn the artificial agents are more likely to be used, even though there is more consequence in negative wastes and emissions.
The negative environmental effects of antimicrobial textiles should be considered with their positive impacts as well. Antimicrobial textiles play an important role in modern use by providing a barrier to microbial and fungal growth in clothing. This is widely important, since bacterial growth can occur in dark and moist environments. Antimicrobial textiles play an important role in healthcare both in hospitals and around the home for practical, everyday use. In addition to stopping bacterial growth, they also minimize odors and reduce stains. This means less washes, which in turn reduces water waste. Their prolonged use without washing means less detergent and other chemical waste into water streams as well.
However, the process of creating the antimicrobial textile and its disposal often includes waterborne and airborne wastes, in addition to the leaching that occurs when it goes to a landfill. Together, these cause harm to both the environment and human health. As previously referenced, each specific antimicrobial agent has its own set of wastes and emissions, and the amount of wastes and emissions is dependent on the production and care to be cautious about outputs. For nonbiodegradable antimicrobial agents such as silver nanoparticles and Triclosan, more measures need to be taken to remove them from water waste. If leached into water, silver nanoparticles have been shown to be lethal to small fish10. In order to be safe for disposable, antimicrobial agents such as silver nanoparticles and Triclosan must go through a transformation process to make them degradable; this would minimize their waterborne waste and toxicity2. In addition to the disposal wastes from metal or other synthetic antimicrobial agents, there is a large amount of wastes and emissions that result from the process of applying the textiles to the fibers. In a physical process, the antimicrobial agents can be applied during spinning, extrusion, or during the finishing process4. However, these processes would involve similar wastes and emissions to any factory; these would include toxic waste water, airborne exhaust, and chemical residue as a result from the production of the textile.
As previously mentioned, the type of antimicrobial agent affects the amount of energy and effort required to safely and properly dispose of an antimicrobial textile. Synthetic or unnatural antimicrobial agents such as silver nanoparticles are most likely to be leached into the environment if not disposed of properly. Silver nanoparticles can enter the environment either through waste water from washing or from being left in a landfill. Because silver is antimicrobial, its unnatural presence could drastically impact local bacteria populations that occur naturally11. When silver nanoparticles enter the environment from clothes being laundered, they will travel to a water purification facility, however the treatment systems are often not equipped to remove nanoparticles. This leads to either nanoparticles remaining in purified water or the leftover bio-sludge, which usually is repurposed as fertilizer for agriculture11. Nanoparticles left in fertilizer will eventually leach back into ground water or rivers. In addition, soil that has been contaminated with silver nanoparticles has the potential to kill the beneficial microbes and fungi that is needed for agricultural growth. Silver nanoparticles have the potential to harm full ecosystems. In addition to silver nanoparticles being found in fertilizers, particles left in landfills can leach into ground water sources as well, resulting in more contamination of water sources.
In conclusion, antimicrobial textiles are an innovative way of fighting bacteria in clothing, however, the threat of the damage that its wastes and emissions can cause on the environment and the people that exist within it calls for more extensive research. There is little information about the exact chemicals that can leach into the environment and about how big the problem of silver nanoparticle leaching actually is. The potential for natural antimicrobial agents such as proteins and crustacean waste such as chitosan could be a much better option, however little information exists about it currently. While an increase of domestic antimicrobial textiles is promising, the fibers containing silver nanoparticles should be restricted to environments such as hospitals where the products are in demand right now. If widely used, antimicrobial textiles have the potential to contaminate waste water and soil, which in turn could affect the agriculture industry and the health of humans and animals that consume the food grown in these conditions.
Notes
1. Tanner, Benjamin. “Antimicrobial Fabrics – Issues & Opportunities in the Era of Antibiotic Resistance”. Microchem Laboratory. Applied Technology Article, AATCC Review. Volume 9, Number 11, pages 30-33. Nov 2009
2. Guedes, Rui Miranda, Lopes, Ascensão Maria, and Morris, Diana Santos. “Antimicrobial Approaches for Textiles: From Research to Market.” MDPI Review. 21 June 2016.
3. Shahidi, Sheila, and Wiener, Jakub. “Antimicrobial Agents in Textile History”. InTech. 2007.
“Safer Than Silver: Antibacterial Material Made With Algae”. KTH Royal Institute of Technology. 1 October 2014.
4. Admin. “Antimicrobial Technology. Antimicrobial Additives. Biocote Ltd.” BioCote, BioCote, 5 Mar. 2018, www.biocote.com/.
5. Resistex Project. “Textile Waste Minimisation: Alternatives for waste volume reduction in the textile sector through the application of minimisation measures in the production process and in the consumption”. Restistex Project. 2007.
6. McMahon, Jeff. “New Antibacterial Clothing: Secret Ingredient Revealed.” Forbes. 9 July 2011.
7. Farkas, S., Piett, C., Syed, NI., Qazzaz, M., and Xu, F. “Silver Nanoparticles (AgNPs) Cause Degeneration of Cytoskeleton and Disrupt Synaptic Machinery of Cultured Cortical Neurons.” Mol Brain. PubMed.gov. 2013 June 19.
8. Robert B. Reed et al. “Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles.”Environmental Science and Technology. 2016.
9. Lim, Sang-Hoon and Hudson, Samuel. “Review of Chitosan and its Derivatives as Antimicrobial Agents and Their Uses as Textile Chemicals”. Journal of Macromolecular Science Part C Polymer Reviews. Jan 2003.
10. Uddin, Faheem. “Environmental Concerns in Antimicrobial Finishing of Textiles”. International Journal of Textile Science. 2014.
11. Lohse, Sam. “Nano Contaminants: How Nanoparticles get into the Environment”. Nanoparticles and the Environment Series, Pt. 1. Center for Sustainable Technology. May 2014.
Bibliography
Admin. “Antimicrobial Technology. Antimicrobial Additives. Biocote Ltd.” BioCote, BioCote, 5 Mar. 2018, www.biocote.com/.
American Chemical Society. “The Impact of Anti-Odor Clothing on the Environment”. ACS News Service Weekly PressPac. 30 March 2016.
Cao, Huiliang. “Silver Nanoparticles for Antibacterial Devices: Biocomparibility and Toxicity”. Boca Raton, FL: CRC Press. 2017.
Farkas, S., Piett, C., Syed, NI., Qazzaz, M., and Xu, F. “Silver Nanoparticles (AgNPs) Cause Degeneration of Cytoskeleton and Disrupt Synaptic Machinery of Cultured Cortical Neurons.” Mol Brain. PubMed.gov. 2013 June 19.
Guedes, Rui Miranda, Lopes, Ascensão Maria, and Morris, Diana Santos. “Antimicrobial Approaches for Textiles: From Research to Market.” MDPI Review. 21 June 2016.
Lim, Sang-Hoon and Hudson, Samuel. “Review of Chitosan and its Derivatives as Antimicrobial Agents and Their Uses as Textile Chemicals”. Journal of Macromolecular Science Part C Polymer Reviews. Jan 2003.
Lohse, Sam. “Nano Contaminants: How Nanoparticles get into the Environment”. Nanoparticles and the Environment Series, Pt. 1. Center for Sustainable Technology. May 2014.
McMahon, Jeff. “New Antibacterial Clothing: Secret Ingredient Revealed.” Forbes. 9 July 2011.
Prevention and Zerbe, Leah. “Germ-Resistant Clothes: Pick or Pass?”. Abcnews. 11 March 2012.
Resistex Project. “Textile Waste Minimisation: Alternatives for waste volume reduction in the textile sector through the application of minimisation measures in the production process and in the consumption”. Restistex Project. 2007.
Robert B. Reed et al. “Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles.”Environmental Science and Technology. 2016.
Shahidi, Sheila, and Wiener, Jakub. “Antimicrobial Agents in Textile History”. InTech. 2007.
“Safer Than Silver: Antibacterial Material Made With Algae”. KTH Royal Institute of Technology. 1 October 2014.
Thomas, Pat. “Antibacterial Clothing-a Fashionable Threat to Human Health: Natural Health News. 29 December 2011.
Tanner, Benjamin. “Antimicrobial Fabrics – Issues & Opportunities in the Era of Antibiotic Resistance”. Microchem Laboratory. Applied Technology Article, AATCC Review. Volume 9, Number 11, pages 30-33. Nov 2009
Uddin, Faheem. “Environmental Concerns in Antimicrobial Finishing of Textiles”. International Journal of Textile Science. 2014.