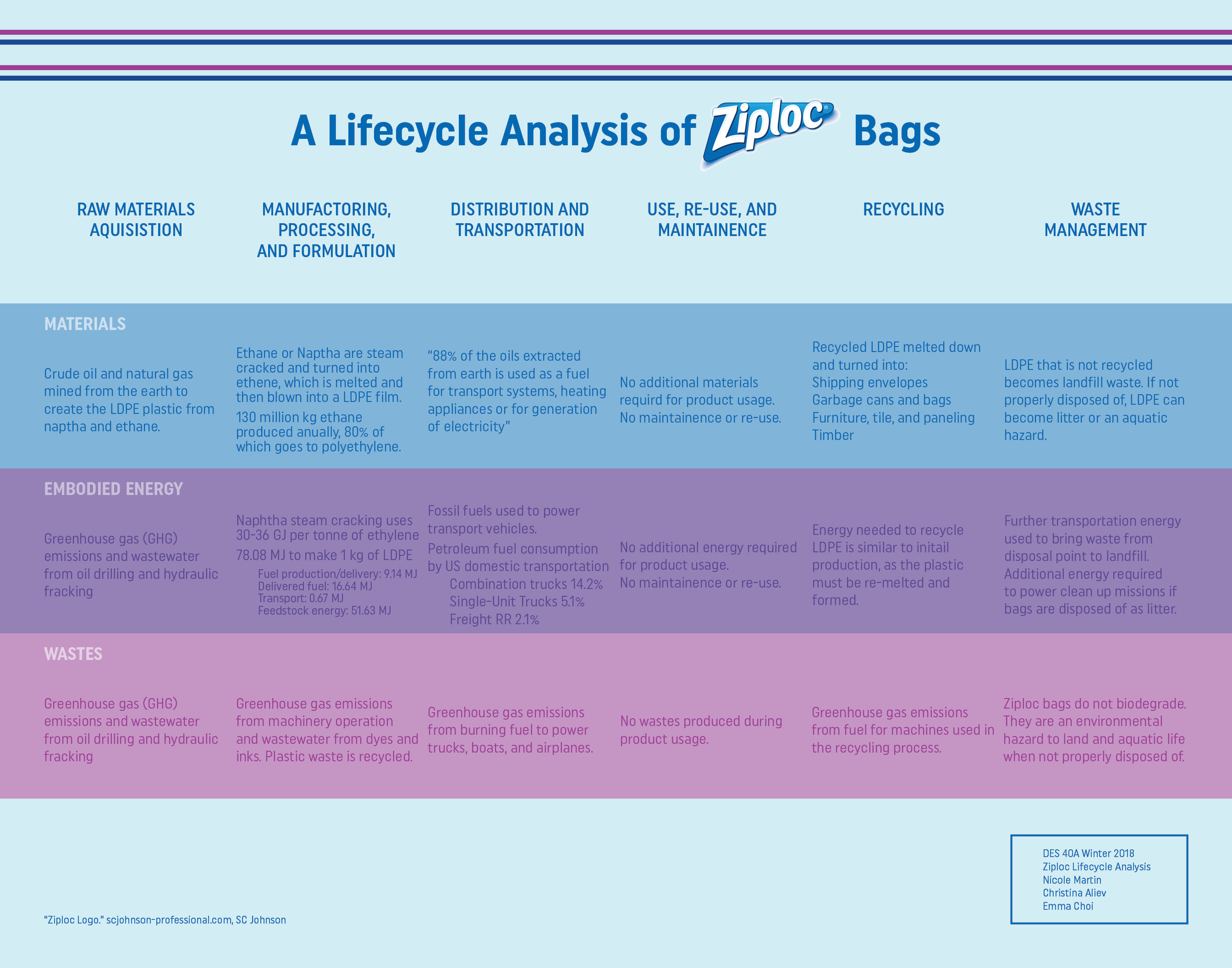
Design Life-Cycle
assess.design.(don't)consume
Nicole Martin
Professor Christina Cogdell
Design 40A
15 March, 2018
An Analysis of the Wastes and Emissions Throughout the Lifecycle of Ziploc® Bags
The Ziploc® bags produced by SC Johnson and their off-brand counterparts have become staples of the Western kitchen. The average United States family uses 500 Ziploc bags each year (Bennett). With 325,719,178 people living in the United States as of July 2017, and an average family size of 2.8 people (U.S. Census Data), that means that the United States alone uses an average of 58,164,139 Ziploc bags per year. The rapid consumption of single-use products like the Ziploc bag is a primary contributor to the growing masses of garbage both in designated landfills and as uncollected litter and ocean rubbish. Further, the processes involved in making Ziploc bags contribute to the annual emissions of greenhouse gasses (GHGs), as well as other contaminants into the atmosphere and environment.
Ziploc bags are made of low density and linear low density polyethylene film (LDPE) a common plastic product with many industrial applications. Like most plastics, LDPE is produced from fossil products. LDPE is created through high-pressure polymerization of ethene, an olefin produced from ethane or naphtha, which are derived from natural gas and crude oil respectively. Both natural gas and crude oil are excavated from the earth through drilling processes. There are two primary types of drilling, referred to as conventional and unconventional drilling. Conventional drilling involves building a well, drilling straight down, and retrieving oil using a lift. Unconventional drilling was developed as conventional drilling rigs began to run dry. This method involves hydraulic fracking, shooting high pressure water mixed with toxic chemicals and sand horizontally through an oil well to induce fracturing in the earth that releases previously inaccessible pockets of oil or natural gas. Unconventional drilling has become more common as natural gas and oil deposits have been depleted, and the remaining resources have become more difficult to access through conventional drilling.
Although both drilling methods have the potential to cause environmental harm, unconventional drilling is far more hazardous. “Studies have shown that approximately
3.6-7.9% of the methane from [unconventional] gas production escapes to the atmosphere in venting and leaks over the lifetime of a well…more than twice as great as [emissions] from conventional gas.” (Finkel). These methane emissions contribute heavily to global warming, as methane has 34 times as much global warming potential (GWP) as carbon dioxide. In addition to releasing large amounts of GHGs into the atmosphere, unconventional drilling is also a water contamination hazard. The wastewater produced by fracking is highly toxic and is often not properly cleaned up by the drillers. “Thirty to seventy percent of the fracking fluid will resurface bringing back with it toxic substances” (Finkel), and it is nearly impossible to trace the chemicals that end up in the surrounding environment after hydraulic fracking. These chemicals contaminate ground and surface water (oftentimes the drinking water of residents) and have been shown to have a negative effect on nearby wildlife, causing harm including low fertility and death. (Finkel).
After the natural gas and oil have been extracted from the earth, they must be further refined in order to separate out the ethane and naphtha used to produce ethene. Ethane is a natural gas liquid (NGL) that is separated out from natural gas along with other NGLs during the refinement process due to the high market value of NGLs as isolated materials. There are two primary methods of separating NGLs from natural gas: the absorption method and the cryogenic expansion process. The cryogenic expansion process is used to extract lighter hydrocarbons like ethane. This process requires reducing the temperature of the natural gas to -120 degrees Fahrenheit, then running the gas at a high-pressure through a turbine which will rapidly expand the gas and allow for the recovery of 90-95% of the ethane contained in the gas stream (NaturalGas.org). Achieving such an extreme temperature on a large production scale requires high-energy consumption which produces carbon dioxide emissions from the combustion of crude oil. Naphtha is produced through the distillation of crude oil, a complex heat intensive process utilizing furnaces, boilers, and gas turbines, which is estimated to produce 32.8 tonnes of carbon dioxide per hour of operation (Gadalla). That’s twice as much carbon dioxide as a single resident of California will produce in one year as of 2012 (CCST). Once separated from their sources, ethane and naphtha are steam cracked to produce ethene. There is an estimated .0112 tonnes of carbon dioxide released for every tonne of ethene produced through steam cracking (Ren).
After steam-cracking, ethylene (ethene in its gaseous state) is converted into a solid by heating it at high temperatures in a low-oxygen chamber. This produces LDPE in the form of small white pellets, which are then melted down and hit with high-pressure air in a process called “blowing” that creates LDPE film. This process is typically fueled by crude oil or natural gas, leading to further GHG emissions in the form of burned fuel.
Water-based ink and red and blue dye pigments are used to create a write-on label on the bag and to add color to the zip closure. Ziploc brand bags use Clariant brand dye pigments and a water-based ink produced by Siegwerk. In 2017 Clariant generated .97 million metric tonnes of GHG emissions and 13 million cubic meters of wastewater (Natural Capital - Clariant Integrated Report 2017). As this is the waste generation of an entire company, the wastes associated with the production of dyes for Ziploc bags is likely a fraction of these numbers. Siegwerk does not have any specific numbers associated with their waste production, but do state that they are committed to reducing their production of hazardous waste by 20% by 2020 (Siegwerk's Environmental Performance) (although it is unclear what their starting number is). The hazardous waste to which Siegwek refers is likely large amounts of toxic wastewater, a byproduct of water-based ink production which is difficult to treat, and must be handled carefully to avoid environmental contamination.
The assembly of the Ziploc bag is carried out by SC Johnson at one of their factories. They have many production plants around the globe, including several in the United States, Netherlands, and China. 32.7% of SC Johnson’s energy comes from renewable sources (Sustainability at SC Johnson). They primarily use wind energy, but also use solar energy and biofuel. SC Johnson has also established a zero-waste program and have less than .5 kg of manufacturing waste per 100kg of product shipped (this number pertains to all SC Johnson products, not specifically Ziploc bags), and have reduced global greenhouse gas emissions by 51.7% since 2000. (SC Johnson Sustainability Report). These are fairly significant strides towards sustainability, however, the practices carried out by SC Johnson are only part of the story of a Ziploc bag. Although SC Johnson requires that suppliers of materials follow proper disposal laws for any hazardous materials, they only “encourage” the monitoring of carbon footprint and a commitment to improve environmental performance. These supplier guidelines do not eliminate most of the wastes produced by LDPE, as the hefty wastes produced by processes such as oil drilling and steam cracking are currently completely legal.
The manufacture and sale of Ziploc bags is a multi-continental process. SC Johnson, Clariant, Siegwerk, and the world’s major oil and plastic companies all have locations across multiple countries and continents. It is unlikely that the entire Ziploc production process is conducted in one country. Ziploc bags most likely make at least one trip around the world in their production, and then must be transported to their sale destination from one of SC Johnson’s factories. This transport, whether it is by freight, boat, or aircraft, is powered by fossil fuel or natural gas, and will have carbon emissions associated with the journey. However, given that Ziploc bags are quite small and light, on a large enough transport scale, the presence of Ziploc bags will not contribute heavily to the carbon emissions from the journey.
It is possible to re-use Ziploc bags, provided that one cleans and dries the bag before using it a second time. In fact, there are even some products dedicated to holding Ziploc bags open for effective drying. However, given that this practice is not particularly common, nor specifically endorsed by SC Johnson, Ziplocs are effectively single-use products.
SC Johnson claims that recycling Ziploc bags is as easy as using them. “Yes, it’s true, Ziploc® brand bags are recyclable. Really! Just look for the bin next time you’re at your local participating store. Your used Ziploc® brand bags (clean and dry) go in the same bins as those plastic shopping bags.” (Sustainability & Safety | Ziploc® Brand) While it’s great that the company is promoting the responsible disposal of their product, realistically, a Ziploc bag’s journey from the home to the recycling plant is much more convoluted. LDPE film is categorized as a #4 plastic by United States recycling systems. Recycling #4 plastic is, unfortunately, not as easy as recycling things like cardboard and paper. Not all curbside collection accepts #4 plastic, and of those that do only some accept LDPE film like Ziploc and other plastic bags, while many only accept solid #4 plastics such as milk cartons. Taking Ziploc bags to the grocery store is an option, and many popular chains have collection programs (although not in all locations), however this requires keeping one’s own collection of cleaned and dried bags and remembering to take them to the store. These recycling programs do not offer compensation like plastic bottle and aluminum can recycling programs do, so there is little incentive for the consumer to put in the extra effort to clean, dry, store, and transport a bag rather than just chuck it in their trash bin.
If Ziploc bags do make it to the recycling plant, there are some limitations as to how the LDPE can be recycled. The plastics must be sorted, cleaned, melted down and then re-extruded into plastic pellets. This process is more expensive than making LDPE from new materials, as it includes the extra costs and labor of sorting and cleaning the used material. USDA law prohibits the use of reclaimed LDPE in any packaging that is to come into direct contact with food. As food packaging is a common use for LDPE film, recycled LDPE will often become hard plastic products instead. Common uses for recycled LDPE include garbage cans and trash bags, furniture, and shipping materials. When not recycled, Ziploc bags pose a serious threat to the environment. Like all plastics, Ziplocs can cause harm to aquatic life if they make their way into a freshwater or marine environment. In a landfill environment, Ziploc bags do not decompose, so every bag tossed into a landfill stays there forever.
The use and production of Ziploc bags is both unethical and unsustainable. The plastics, dyes, and inks used in Ziploc bags come from two of the world’s most polluting industries, and their production shows no regard for the need to reduce landfill waste and greenhouse gas emissions. Ziploc bags create environmental damage throughout their entire lifecycle and at the end of their lifecycle, ultimately become waste themselves, as we do not have the infrastructure to effectively recycle the bags on the same scale that they are being consumed. Although
SC Johnson has made efforts to reduce greenhouse gas emissions and waste in their manufacturing process, they are still producing a single-use product with zero biodegradation and limited recycling accessibility.
Works Cited
Bennett, Sophia. “How to Recycle Ziploc Bags.” RecycleNation, ERI, 7 Oct. 2014, recyclenation.com/2014/10/recycle-ziploc-bags/.
CCST, “California's Energy Future: The View to 2050.” California Council on Science and Technology, 2011, California's Energy Future: The View to 2050, ccst.us/publications/2011/2011energy.php.
Finkel, M.l., and J. Hays. “The Implications of Unconventional Drilling For Natural Gas: a Global Public Health Concern.” Public Health, vol. 127, no. 10, Oct. 2013, pp. 889–893. Science Direct, Elsevier, doi:10.1016/j.puhe.2013.07.005.
Gadalla, M, et al. “Estimation and Reduction of CO2 Emissions from Crude Oil Distillation Units.” Energy, vol. 31, no. 13, 2006, pp. 2398–2408. Science Direct, Elsevier, doi:10.1016/j.energy.2005.10.030.
Goodship, Vannessa. “Plastic Recycling.” Science Progress, vol. 90, no. 4, 2007, pp. 245–268. Science Reviews 2000, JSTOR, doi:10.3184/003685007x228748.
Infoplease, Infoplease, www.infoplease.com/us/household-and-family-statistics/us-households-size-1790-2006.
IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp, doi:10.1017/CBO9781107415324.
Johnson, SC. “Ziploc® Brand Freezer Bags : SC Johnson.” SC Johnson - What's Inside, www.whatsinsidescjohnson.com/us/en/brands/ziploc/ziploc-brand-freezer-bags.
Kant, Rita. “Textile Dyeing Industry an Environmental Hazard.” Natural Science, vol. 04, no. 01, 2012, pp. 22–26. Scientific Research, SCRIP, doi:10.4236/ns.2012.41004.
Lazonby, John. “Poly(Ethene) (Polyethylene).” The Essential Chemical Industry, Centre for Industry Education Collaboration (CIEC), 27 Apr. 2017, www.essentialchemicalindustry.org/polymers/polyethene.html.
Lee, Jessica. “Conventional Vs. Unconventional.” Croft Systems Oil & Gas Blog, Croft Production Systems, 25 June 2015, www.croftsystems.net/oil-gas-blog/conventional-vs.-unconventional.
Lepoutre, Priscilla. “The Manufacture of Polyethylene.” New Zealand Institute of Chemistry, www.nzic.org.nz/ChemProcesses/polymers/10J.pdf.
Liptow, Christin, and Anne-Marie Tillman. “A Comparative Life Cycle Assessment Study of Polyethylene Based on Sugarcane and Crude Oil.” Journal of Industrial Ecology, vol. 16, no. 3, 2012, pp. 420–435. Wiley Online Library, Wiley, doi:10.1111/j.1530-9290.2011.00405.x.
MetesÌ, A, et al. “The Role of Zeolites in Wastewater Treatment of Printing Inks.” Water Research, vol. 38, no. 14-15, 2004, pp. 3373–3381. Science Direct, Elsevier, doi:10.1016/j.watres.2004.04.012.
“Natural Capital - Clariant Integrated Report 2017.” Reports.clariant, Clariant, reports.clariant.com/2017/integrated-report/review-of-results/planet/natural-capital.html.
“NaturalGas.org.” NaturalGasorg, naturalgas.org/naturalgas/processing-ng/.
“Reducing Resource Use | Using Renewable Energy | Sustainability at SC Johnson.” Sjjohnson, SC Johnson, web.
Ren, Tao, et al. “Steam Cracking and Methane to Olefins: Energy Use, CO2 Emissions and Production Costs.” Energy, vol. 33, no. 5, May 2008, pp. 817–833. Science Direct, Elsevier, doi:10.1016/j.energy.2008.01.002.
Ross, Stuart, and David Evans. “The Environmental Effect of Reusing and Recycling a Plastic-Based Packaging System.” Journal of Cleaner Production, vol. 11, no. 5, Aug. 2003, pp. 561–571. Science Direct, Elsevier, doi:10.1016/s0959-6526(02)00089-6.
“SC Johnson Supplier Code of Conduct.” Scjohnson, SC Johnson, web.
“SC Johnson Sustainability Report.” Scjohnson, SC Johnson, web.
“Siegwerk's Environmental Performance.” Siegwerk Druckfarben AG & Co. KGaA, Siegwerk, 13 Mar. 2018, www.siegwerk.com/en/our-responsibility/siegwerks-environmental-performance.html.
“Sustainability & Safety | Ziploc® Brand.” Ziploc ®, ziploc.com/en/sustainability-and-safety.
Emma Choi
Professor Christina Cogdell
DES 40A
15 March 2018
Ziploc Bags: Embodied Energy
Ziploc bags are a fairly common product: after all, the average American family uses 500 Ziploc bags per year (Bennett). Yet, despite their popularity, not many people likely know what goes into producing such a product; after all, the life cycle of any product is not often taken into consideration by the majority of the population. The production of Ziploc bags is an energy intensive process with different possible alternatives that may reduce the energy consumption.
Ziploc bags are composed primarily of low density polyethylene (LDPE) and linear low density polyethylene (LLDPE), plastics derived from ethene (SC Johnson, Lazonby). Ethene itself comes from ethane, which is present in natural gas. As traditional gas supplies decline, hydraulic fracturing, or fracking, of shale gas is becoming a more popular source of natural gas (Clegg). Fracking allows users to obtain gas and oil from impermeable shale rock that was previously unattainable when using conventional drilling. It takes two or more kilometers of drilling vertically to reach the shale, then additional horizontal drilling, up to three kilometers. Slickwater (a mixture of water, sand, and additives) is pumped through the well pipe at high pressure (greater than 600 atmospheres). The horizontal section of the well pipe contains small perforations, creating microfractures in the shale; the proppants, or solid materials (like sand), in the slickwater prop open these fractures, allowing the gas and oil to flow freely from the rock. The gas and oil are captured by a well head once the pressure is released (Lichtarowicz).
Fracking is clearly dependent on water, which begs the question: how much water does this process use? While this depends on the type and location of the well, a single well requires 2.3 to 3.8 million gallons (8.7 to 14.4 million liters) of water. In 2005, the water used for mining accounted for less than 1.0% of the water withdrawn in the US; compared to thermoelectric power generation (49%) and irrigation (31%), this number seems fairly insignificant. While this may be true in the big picture, 2 million gallons (7.6 million liters) per well can often be significant for the area the water is extracted from, as these areas tend to be remote and environmentally sensitive (Chen). In comparison to conventional gas drilling, fracking requires more water. However, using natural gas to generate electricity saves water compared to coal; coal consumes more than twice the amount of water per megawatt hour generated than unconventional gas. In comparison to renewable energies, fracking may use more or less depending on the source: fracked gas uses less than 0.01% of water per unit energy than corn ethanol, but solar and wind use almost no water (Golden).
After obtaining natural gas comes the extraction of natural gas liquids (NGL) like ethane, propane, butanes, and natural gasoline (the main focus here will be ethane). There are two different methods of extracting NGLs: absorption and cryogenic expansion. In the absorption method, the natural gas is passed through an absorption tower, where it comes in contact with “lean” absorption oil. The oil absorbs much of the NGLs, then exits the tower, and the now “rich” absorption oil goes to a lean oil still. Here, the oil-NGL mixture is heated at a point between the boiling point of the NGL and the oil. This yields approximately 75% of the butanes and 85 to 90% of the natural gasoline. The process can be improved to target specific NGLS by refrigerating the lean oil before it enters the absorption tower; this gives a yield of approximately 90% of the propane, 40% of the ethane, and nearly 100% of the natural gasoline. While absorption is good for heavier NGLs, lighter NGLs, like ethane, have a lesser recovery rate. A more effective extraction process is cryogenic expansion, which requires lowering the gas stream to about -120 degrees Fahrenheit. The most effective way of doing this is the turbo expander process, which cools the gas with external refrigerants, followed by an expansion turbine. This expands the gas very rapidly, resulting in a great and rapid temperature drop; hydrocarbons like ethane condense, while methane remains gaseous. This process yields an ethane recovery of 90-95%, and even saves energy costs: the expansion turbine can convert energy from the expansion of the gas into recompressing methane (Maverick Engineering).
Once ethane is obtained, it is converted to ethene by steam cracking, the most energy-consuming process in chemical processing (Ren). Reactant gases (ethane, propane, butane) or liquids (naphtha, gas-oil) are preheated and vaporized; then, in a tubular reactor, they are mixed with steam then heated to 1050-1150 K and converted to low relative molecular mass alkenes. There are a variety of possible feedstocks, and each produces different product yields. Ethene is usually the highest percentage yield product compared to other products, but has the highest yield (78%) from an ethane feedstock (Lichtarowicz). A 1985 energy analysis by the US Department of Energy measured the specific energy consumption of cracking ethane, with a 24% contribution to steam, heating, and losses; this was approximately equal to the other sections (heat of reaction, fractionation/compression, and separation). In the cracking of naphtha, an exergy (usable energy) analysis of an ethylene process from the Tamkang Journal of Science and Engineering attributed de-ethanization (fractionation of ethane) with 23% of exergy loss in compression and separation; this was the second highest loss (to 30% in propylene refrigeration). Perhaps this is due to a greater amount of ethane, but is regardless a significant part of energy usage (Ren).
Finally, ethene is used to produce polyethylene. There are three types of polyethylene: low density (LDPE), linear low density (LLDPE), and high density (HDPE); as stated before, Ziplocs consist of LDPE and LLDPE (Lazonby, SC Johnson). Polyethylene accounts for over 60% of ethene manufactured per year, and is the world’s most important/used plastic. The annual global production of LDPE in 2014 was 18.7 million tonnes, and 24.1 million tonnes of LLDPE. The production of LDPE occurs under extreme pressure (1000 to 3000 atm) at moderate temperatures (420-570 K). Ethene with a purity in excess of 99.9% is compressed, then passed to a reactor with an initiator (eg. small amount of oxygen); molten polyethylene is then removed, extruded, and cut into granules, while unreacted ethene is recycled (Lazonby). A report on the production of 4.48 million tonnes of LDPE (representative of 93.5% of Western Europe production) found the average gross energy to produce one kilogram of LDPE was 77 megajoules (range from 64 to 96 megajoules) (Boustead). Producing LLDPE with high pressure is costly, so improved techniques using catalysts has been developed. Slurry, solution, or gas phase processes can be used with a Ziegler-Natta catalyst, while inorganic catalysts require the use of the gas phase process. The slurry process polymerizes ethene by passing it under pressure into the slurry; granules of Ziegler-Natta catalyst mixed with a liquid hydrocarbon, a diluent, are also in the reaction, which occurs in a loop reactor. Once the product is released, the solvent evaporates, leaving the polymer while leaving the catalyst; to destroy the catalyst’s activity, water vapor reacts with the catalytic sites. The solution process passes ethene and hydrogen under pressure into a Ziegler-Natta catalyst and hydrocarbon solution, obtaining polyethylene in a similar manner to the slurry process. The gas phase process passes ethene and hydrogen over a Phillips catalyst in a fixed bed reactor, resulting the ethene to polymerize into grains that pass out of the reactor once the valve is released. LLDPE is ideal for film products because the material is quite resilient and flexible without needing plasticisers (Lazonby). While I could not find specific numbers for the production of LLDPE, production of LLDPE is greater than that of LDPE, so assumedly LLDPE production uses more energy than that of LDPE.
Ziploc bags also use dye, as notable in their distinctive colored zipper; one side is red and the other is blue, thus when closed the bag shows purple. The dyes, red and blue, used are from the company Clariant (SC Johnson). Clariant’s report on Natural Capital states that their total energy consumption increased 10.0% from 2,950 to 3,245 kWh/t from 2016 to 2017. However, they cite the energy consumption of production as decreasing by 1.8% from 719 to 706 kWh/t. Clariant attributes the increase in total energy consumption to increasing production volume. Clariant has goals to decrease their energy consumption by 30% by 2025 (Clariant). Thankfully, they seem to be mindful of reducing energy consumption, and are perhaps on their way there. Ziploc bags also incorporate water-based ink (SC Johnson). However, I could not find further information on this. Regardless, this should add further energy consumption costs to production.
SC Johnson, the company that makes Ziploc bags, sites their Bay City, Michigan plant as producing Ziploc bags; in 2011, 67% of the plant’s energy was supplied by a nearby wind farm (SC Johnson). In 2017, the company announced it was expanding, also including that the plant produces billions of bags per year, distributing them to seven different countries (The Associated Press). I could not find more information about this, but in terms of transportation, a study for the US Department of Energy provided information of petroleum fuel consumption by US domestic transportation (2005/2007). With the majority of petroleum fuel consumption came from cars and light trucks, 14.2% was combination trucks, 5.1% for single-unit trucks, and 2.1% for freight railroads. Altogether, these do not amount to nearly as much as cars and light trucks (approximately ⅔ of consumption) (Cambridge Systematics). However, the study only takes domestic transportation into account, meaning that total petroleum consumption of transportation must amount to more; in this case, SC Johnson claims they distribute Ziploc bags to seven different countries, thus amounting to further consumption due to transportation, but on an international scale.
Transportation considerations can be similarly applied to waste, as assumedly waste must be transported to various waste management sites. Unfortunately, Ziploc bags are generally single-use products, as reusing them requires cleaning and caring for them (Bennett). I do not believe this process is utilized by many people, as Ziploc bags are widely seen and advertised as a convenient disposable product. Ziploc’s sustainability and safety page claims the majority of plastic bags are made into composite lumber, which is used for various products including fences, benches, and decks; TREX, Hilex, and AERT are sited as “key plastic recycling organizations” (SC Johnson). I continued further investigation of one of these organizations, TREX, which gave me some information on their usage of recycled Ziploc bags. Cleaned plastic waste is ground into granules, which is then combined with sawdust and heated. TREX claims they do not use smokestacks and recycle any waste made back into manufacturing (however, they do not describe how), claiming they produce “virtually zero waste” in manufacturing. In addition to composite lumber products, they also produce LLDPE pellets with surplus material, claiming they are made from 100% recycled materials (Trex). While this all sounds incredibly eco-friendly, claims of “virtually zero waste” and products made from “100% recycled materials” are quite suspicious. However, this report is not on composite lumber or Trex, so no there will be no further research in this paper. Ultimately, it is positive that Ziploc bags are being utilized in another way before they reach the end of their lifecycle.
Ziploc bag production utilizes some controversial and incredibly energy intensive processes (fracking and steam cracking) and are mass produced, mass distributed single-use products. As such, there is constant production, and as the population increases, so does demand, meaning even more production. To reduce energy consumption, people should try to reduce their dependence on Ziploc bags and perhaps consider using reusable storage containers.
Works Cited
Bennett, Sophia. "How to Recycle Ziploc Bags." RecycleNation. N.p., 07 Oct. 2014. Web. 15 Mar. 2018.
Boustead, I. "Eco-profiles of the European Plastics Industry." PlasticsEurope, Mar. 2005. Web. 15 Mar. 2018.
Cambridge Systematics. "Freight Transportation Modal Shares: Scenarios for a Low-Carbon Future." US Department of Energy, Mar. 2013. Web. 15 Mar. 2018.
Chen, Jiangang et al. “Hydraulic Fracturing: Paving the Way for a Sustainable Future?” Journal of Environmental and Public Health 2014 (2014): 656824. PMC. Web. 15 Mar. 2018.
Clariant. "Natural Capital." Clariant. Clariant, n.d. Web. 15 Mar. 2018.
Clegg, Brian. "Ethane." Chemistry World. N.p., 02 Oct. 2014. Web. 15 Mar. 2018.
Lazonby, John. "Poly(ethene) (Polyethylene)." The Essential Chemical Industry Online. N.p., n.d. Web. 15 Mar. 2018.
Lichtarowicz, Marek. "Cracking and Related Refinery." The Essential Chemical Industry Online. N.p., 7 Sept. 2014. Web. 15 Mar. 2018.
Lichtarowicz, Marek. "Extracting Crude Oil and Natural Gas." The Essential Chemical Industry Online. N.p., 3 July 2017. Web. 15 Mar. 2018.
Maverick Engineering. "Oil & Gas: Natural Gas Processing: NGL Processing." Maverick Engineering. Maverick Engineering, Inc., n.d. Web. 15 Mar. 2018.
Ren, Tao, et al. “Olefins from Conventional and Heavy Feedstocks: Energy Use in Steam Cracking and Alternative Processes.” Energy, Pergamon, 17 May 2005. Web. 15 Mar. 2018.
SC Johnson. "Clean Energy and SC Johnson." SC Johnson Fact Sheets. N.p., n.d. Web. 15 Mar. 2018.
SC Johnson. "Sustainability & Safety | Ziploc® Brand." Ziploc ®. SC Johnson, n.d. Web. 15 Mar. 2018.
SC Johnson. "Ziploc® Brand Freezer Bags : SC Johnson." SC Johnson - What's Inside. N.p., n.d. Web. 15 Mar. 2018.
Trex. "ECO-FRIENDLY DECKING." Trex. Trex Company, Inc., n.d. Web. 15 Mar. 2018.
Trex. "VIDEO GALLERIES." Trex. Trex Company, Inc., n.d. Web. 15 Mar. 2018.
Trex. "TREX® SPECIALTY MATERIALS." Trex. Trex Company, Inc., n.d. Web. 15 Mar. 2018.
Christina Aliev
Professor Christina Cogdell
Design 40A
22 March, 2018
The Raw Materials and Lifecycle of Ziploc® Bags
Ziploc® bags are used in the day-to-day lives of many, but most individuals do not think about the life cycle of this product. Plastic bags are becoming more relevant to the public for environmental issues since plastics take nearly a thousand years to decompose. On average, the American family uses around 500 bags a year, and most do not reuse bags. The American population is around 326,128,671 (U.S. Population [LIVE]) with an average family-size of 3 family members (Statista), meaning around 54,354,778,500 Ziploc® bags are used yearly, and that number is steadily increasing. The production of this large amount of Ziploc® bags causes environmental issues ranging from greenhouse gas emissions, climate change, and the use of limited raw materials. The creation of Ziploc® bags is complicated because each of the materials needs to be chemically processed. The raw materials in Ziploc® bags are initially extracted from crude oils. From this primary raw material, ethane is then steam cracked into ethylene which is the material used to form polyethylene -- from this, polyethylene low density (LDPE) and polyethylene linear low density (LLDPE) are created and these are the two major materials in Ziploc® bags. After that, Clariant dyes are added for the closure and write-on part of the Ziploc®.
The two major materials in Ziploc® bags are polyethylene low density (LDPE) and polyethylene linear low density (LLDPE) which are extracted from crude oils. Both LDPE and LLDPE are made from polyethylene, a durable and lightweight plastic that is used in many different applications ranging from various plastic parts, laminates, films, packaging, electrical, and automotives (Polyethylene [PE] - Complete Guide). According to the website Omnexus, polyethylene “is one of the most widely produced plastics in the world… tens of millions of tons are produced worldwide each year.” Polyethylene is extracted from crude oils, and there are two different ways this can be done--either through modifying a natural gas (a methane, ethane, or propane mix) or through catalytic cracking of crude oil to gasoline (The Manufacture of Polyethylene). The biggest difference between LDPE and LLDPE is that LLDPE’s molecular structure has shorter branches in its chains. Because of this, the chains can slide against each other more smoothly, making LLDPE stronger and more flexible. Therefore LLDPE has higher tensile strength, higher impact, and higher puncture resistance than LDPE (U.S. Plastic Corporation).
These two materials can actually be found in many products today, not just in Ziploc® bags. It is important to consider where these materials come from because it is an elaborate process to create them. First off, the crude oils that LDPE and LLDPE are extracted from need to be drilled from the earth. There are two different ways that this can be done -- conventional and unconventional drilling. Conventional drilling is when oil and gas is extracted with the natural pressure of the machines, while unconventional drilling involves the machines drilling down horizontally in a process called fracking. Unconventional drilling extracts gas and oils in situations where conventional drilling normally would not be as effective (Lee). In 2016, 65% of the United States oil production came from five major states as follows: Texas at 36% of the nation’s production, North Dakota at 12%, California and Alaska at 6%, and Oklahoma at 5%. Another 18% of the country’s oil came from a well located offshore in the Gulf of Mexico (“Where Our Oil Comes From”). These drilling locations are most likely where most of the crude oils come from for plastic production in the United States. Interestingly, plastic production does not actually consume a very large percentage of oil. According to the organization called PETRA, “a common misconception is that plastics and chemicals are the primary consumers of crude oil. However, combined they account for less than 10% of the global annual usage.”Additionally, I tried researching which specific oil plants the oil that is used for plastic comes from, but I was not successful.
Once the raw crude oils are taken from drilling machines, the goal is to extract ethane and naptha. This is done through steam cracking gaseous or liquid feedstocks. According to Collins Dictionary, steam cracking “is the main method of breaking down large molecules of hydrocarbons, in which a gaseous or liquid hydrocarbon is diluted with steam and then heated.” Although cracking ethane produces little-to-no byproducts, steam cracking naphtha produces a significant amount of propylene, butadiene, and benzene, and according to the American Chemical Society, “globally, steam cracking is the most important source of propylene and butadiene and the second leading source of benzene” (Beyond the Ethylene Steam Cracker). This noncatalytic cracking process is done at extremely high temperatures, usually reaching almost 850 degrees Celsius (Beyond the Ethylene Steam Cracker). This creates ethylene, which is the primary material used to create polyethylene. In order to make polyethylene a usable material for the production of Ziploc® bags, manufacturers convert it to a solid material called resin, which then can be heated again to create LDPE. This can be accomplished because LDPE is a thermoplastic and therefore can be heated and cooled several times in order to mold it into different forms, unlike a thermoset which can only be heated once. The Imperial Chemical Industries states that the first grade of polyethylene was initially produced in 1933 by using a high pressure process via free radical polymerization (Srivastava).
Next, the dyes used in Ziploc® bags according to (SC Johnson) are Clariant PE33760091 red, Clariant PE53760090 blue, hydro film dow EH 70388 and 70393 white inks. The closure on Ziploc® bags has a signature purple color when the bag is sealed, which is a blend of the blue and red-dyed sides of the zipper. White dye is used on bags to create the write-on portion that the consumer can easily label. Siegwerk, the third largest international company that supplies printing inks for packaging applications and labels, provides Ziploc® with the water-based inks (News Herald). Another company that plays a role to the creation of Ziploc® bags is a company named Clariant. Their purpose is to add color to plastic resins and applications. Ziploc® uses Clariant brand dye pigments for the signature red and blue coloring. From the Clariant website, their products: “include REMAFIN® masterbatches for polyolefin polymers, RENOL® masterbatches for high-performance engineering thermoplastics, OMNICOLOR® multipurpose color concentrates, and MEVOPUR® medical-grade masterbatches for the medical industry, delivering hundreds of standard colors as well as custom formulations and color/additive combibatches” (Clariant Ltd). While the exact materials used by the company remains unclear, renol is most likely the dye used to create Clariant PE33760091 red and Clariant PE53760090 blue.
The good thing about the famous Ziploc® bags is that they are recyclable! While this is a positive thing, it is slightly more complicated than just throwing them in a recycling bin at home. The bags need to be properly placed in a single steam recycling location to then be transported to a local Material Recovery Facility (Johnson). Because a typical recycling center uses machines to separate the different materials, the flimsy and lightweight bags can become tangled up in the machines, causing them to be temporarily shut down. Therefore it is vital for Ziploc® bags to be placed in their proper recycle locations, and there are even locations that offer a plastic-bag disposal program to make this easier. The reason being is Ziploc® bags are made from LDPE #4 while PET #1 and HDPE #2 are the main plastics for curbside collection (recyclingbin.com). Another issue is that if the recycled plastic film comes in contact with food again, it becomes a safety issue with the United States Food and Drug Administration Regulations, which then complicates the recycling process for the reuse of Ziploc® bags (Lepoutre). Since the reproduction of recycled plastics can be an intensive process from the transportation to and from the manufacturers, separation of the various materials, reheating/cooling, and molding of the new product from resins, it may be more effective to reuse the Ziploc® bags at home. Not only will this reduce the gas emissions from transportation and cut back on the energy used in a Material Recovery Facility, but it would be more convenient, environmentally friendly, and money saving for the consumer.
Objects that are made from plastic are the root of many environmental issues. One major issue in particular is the enormous amount of plastic sitting in landfills, because plastic items can take hundreds of years to decompose completely. According to a blog entry called “Where do Plastic Bags Go?”, a member of the Environmental Protection Agency, “in 2011, Americans produced around 250 million tons of waste, 32 million tons of that solid waste was plastic. That’s 4.4 pounds of waste per person per day!” (Bond). These numbers are shocking, but it should be noted that emissions from transportation trucks to-and-from facilities weren’t even included in the above figures, so the actual amount is much higher. With that in mind, reducing the amount of single-use objects that go directly to landfills would greatly lessen the amount of waste produced by humans and would help the environment. Even reusing plastic bags and/or Ziploc® bags once can reduce this cycle significantly.
Overall, the production and disposal of Ziploc® bags is harmful to the environment. From attaining the raw materials, to mass production, to distribution/sale, then to usage and eventually to the landfill, the life cycle of Ziploc® bags is a long and complicated process that isn’t beneficial to the planet. The production of Ziploc® bags involve two of the highest world polluting corporations -- being plastics and dyes. More plastic bags are being produced than the amount that can be recycled, so the production of Ziploc® bags will continue to have a negative impact on the environment unless a solution is found that could reduce the amount of crude oil needed to make Ziplocs and/or make the bags either more easily recyclable or compostable.
Works Cited
“Bags | Ziploc® Brand.” Ziploc ®, cid=SEM_G_ziplock%2Bbags.
“Beyond the Ethylene Steam Cracker.” American Chemical Society, www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/beyond-the-ethylene-steam-cracker.html.
Bond, Shannon. “Where Do Plastic Bags Go?” EPA, Environmental Protection Agency, 6 Mar. 2014, blog.epa.gov/blog/2014/03/where-do-plastic-bags-go/.
Clariant Ltd. “Color Masterbatches.” Clariant Ltd., www.clariant.com/en/Business-Units/Masterbatches/Color-Masterbatches.
“ Definition of 'Steam Cracking'.” Collins Dictionary, www.collinsdictionary.com/us/dictionary/english/steam-crackingdictionary/english/steam-cracking.
“Definition of 'Steam Cracking'.” Steam Cracking Definition and Meaning | Collins English Dictionary, www.collinsdictionary.com/us/dictionary/english/steam-cracking.
Hill, Danielle. “What Are Ziploc Brand Bags Made of? | Hunker.” Hunker.com, Hunker, 2 Dec. 2010, www.hunker.com/13419974/what-are-ziploc-brand-bags-made-of.
Johnson. “Don't Trash It: How to Handle Exceptional Recyclables.” MNN - Mother Nature Network, Mother Nature Network, 31 May 2017, www.mnn.com/your-home/at-home/sponsorvideo/dont-trash-it-how-to-handle-exceptional-recyclables.
Lee, Jessica. “Oil & Gas Blog.” Conventional Vs. Unconventional, www.croftsystems.net/oil-gas-blog/conventional-vs.-unconventional.
Lepoutre, Priscilla. “The Manufacture of Polyethylene.” New Zealand Institute of Chemistry, www.nzic.org.nz/ChemProcesses/polymers/10J.pdf.
“Linear Low-Density Polyethylene.” Wikipedia, Wikimedia Foundation, 17 Feb. 2018, en.wikipedia.org/wiki/Linear_low-density_polyethylene.
News Herald. “Siegwerk Brings Color to Marketing.” Morganton.com | The News Herald, 13 May 2014, www.morganton.com/madeinburke/manufacturing/siegwerk-brings-color-to-marketing/article_d7d96f4e-dacb-11e3-a3da-0017a43b2370.html.
“PET – What Is It and Where Does It Come From? .” PETRA, www.petresin.org/pdf/PET_whatisitandwheredoesitcomefrom.pdf.
“Polyethylene (PE) - Complete Guide $Neo(Document), Polyethylene: PE Plastic Advance Guide (HDPE, LDPE, LLDPE...), omnexus.specialchem.com/selection-guide/polyethylene-plastic.
“Professor Plastics: Highlights of Low-Density Polyethylene.” Plastics Make It Possible, 4 Jan. 2018, www.plasticsmakeitpossible.com/about-plastics/faqs/professor-plastics/professor-plastics-highlights-of-low-density-polyethylene/.
Srivastava, Vishal. “How Is LDPE Plastic Made?” Quora, www.quora.com/How-is-LDPE-plastic-made.
“Sustainability & Safety | Ziploc® Brand.” Ziploc ®, ziploc.com/en/sustainability-and-safety.
“What Are the Differences between HDPE, LDPE, XLPE, LLDPE, and UHMWPE?” U.S. Plastic Corporation, www.usplastic.com/knowledgebase/article.aspx?contentkey=508.
“Where Our Oil Comes From.” Where Our Oil Comes From - Energy Explained, Your Guide To Understanding Energy - Energy Information Administration, 8 June 2017, www.eia.gov/energyexplained/index.cfm?page=oil_where.
“Where Our Oil Comes From.” Where Our Oil Comes From - Energy Explained, Your Guide To Understanding Energy - Energy Information Administration, www.eia.gov/energyexplained/index.cfm?page=oil_where.
“Zip Lock Plastic Bags: A Tragic Case of Recycling Inconvenience.” Welcome to Recyclingbin.com, www.recyclingbin.com/Blog/zip-lock-plastic-bags-a-tragic-case-of-recycling-inconvenience.