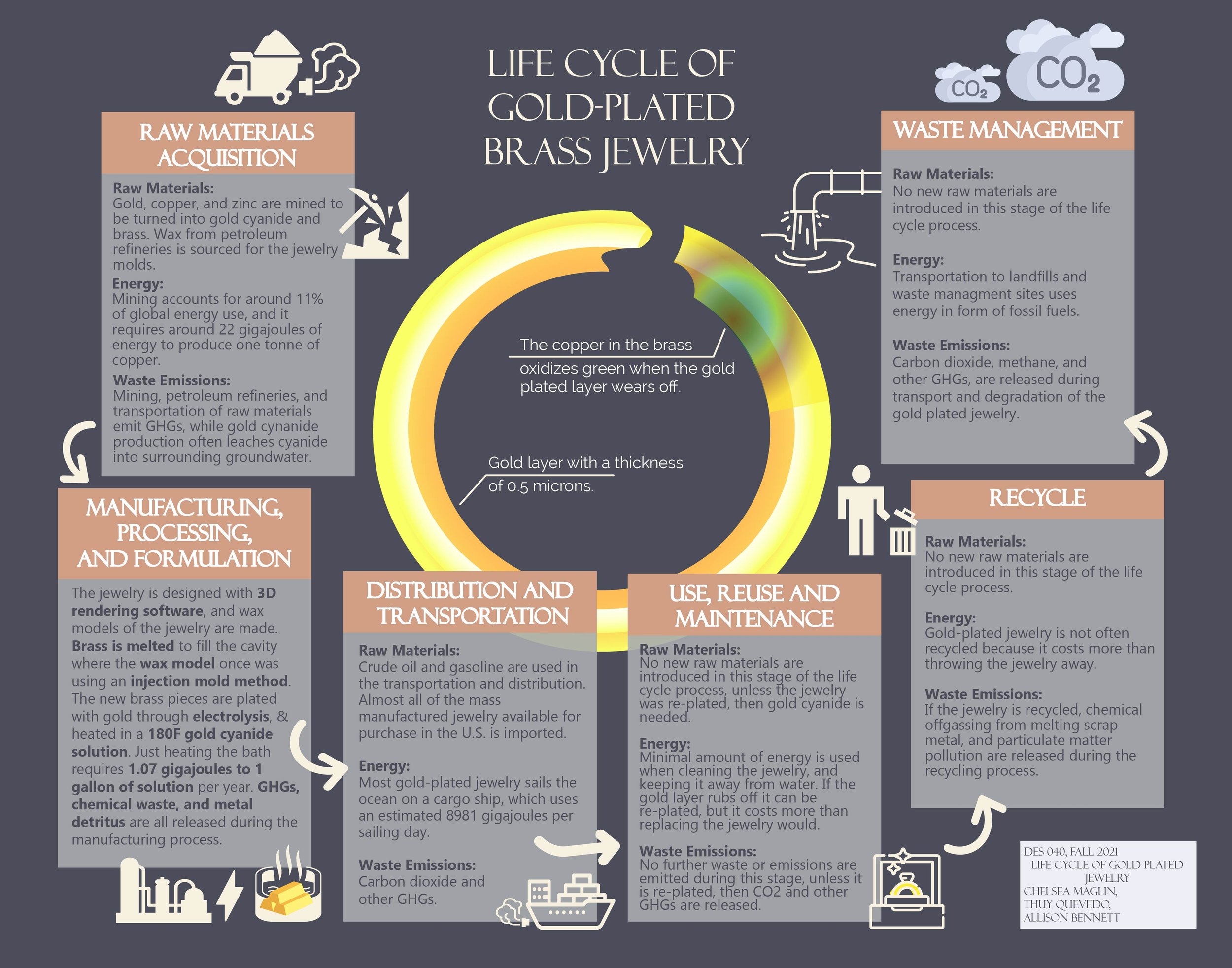
Design Life-Cycle
assess.design.(don't)consume
A Life Cycle Assessment of Gold Plated Brass Jewelry’s Embodied Energy
Thuy Quevedo
Inside of every boutique, or every landfill depending on how you spin it, you can usually find gold-plated jewelry, or what’s left of it. Electroplating small amounts of gold onto cheaper metal alloys has become a staple of the fashion/costume jewelry industry due to the instant yet ephemeral gold colour achieved. Gold-plated fashion jewelry is more accessible to the average consumer because of its low-cost materials and manufacturing. However, the real price of a pair of common gold-plated earrings lies within its embedded (also referred to as embodied) energy, the culmination of energy needed to produce one pair of earrings. The efficiency of mass manufacturing drastically cuts the costs of producing gold-plate jewelry since it can be created and sold in bulk quantities. Yet, to sustain that level of production requires massive amounts of energy from mining the materials to shipping the finished product overseas. This paper shall estimate the embodied energy of certain materials and processes from gold-plated brass jewelry life cycle. There is almost no information about mass manufactured gold-plated jewelry, which is concerning because of its ubiquity. The environmental toll of this overlooked product can only be theorized without proper investigation. The life cycle’s boundaries are set from mining to distribution and the jewelry is manufactured using a lost-wax casting method on an industrial scale: by making wax models of the jewelry, casting brass into the models, and electroplating the brass with gold. From factory to landfill, mass manufactured gold-plated jewelry requires an unsustainable amount of embodied energy throughout the manufacturing and distribution processes, despite the short product lifetime of gold-plated alloys.
At the beginning of the life cycle, factories for gold-plated jewelry must acquire gold, beta brass, microcrystalline wax, and gold cyanide. These are the major materials used in gold-plated brass jewelry, and most of them are secondary materials not raw. The gold-plating process is not unique to the jewelry industry; the technique can be used to attach a thin gold layer to a variety of base metals, but mass-manufactured gold-plated jewelry usually uses an alloy instead of a pure elemental metal. Alloys are a combination of different metals that are mined at the beginning of the life cycle. Gold and zinc are usually “hard rock” mined, a methodology of mining ore underground, whereas copper is primarily “open pit” mined, a surface level mining technique that continually excavates for more ore (“Copper Mining and Processing: Processing Copper Ores”). Mining requires energy in the form of electricity, combustion (explosives), etc. and it’s estimated that mining accounts for 11% of all global energy use (Sykes et al. 8). The embodied energy of copper mining alone is 22.2 GJ/1 tCu, with 2.6 tonnes of CO2 released for every tonne of copper (Sykes et al. 23). Once the copper and zinc has been mined, the two metals are combined to create alpha-beta brass. Alpha-beta brass is an alloy composed of around 40% zinc to 60% copper (Callcut), this secondary material is valued for its strength and casting capabilities and is the base metal of many gold-plated jewelries. The jewelry models are made from microcrystalline wax, another secondary material that is a byproduct of petroleum refinement, an industry that is the “third largest stationary producer of GHG” (Lei et al. 1115) and using estimations from oil refineries, likely needs 0.612MJ per 1 gallon of wax (Palou-Rivera, Wang 9). To plate the brass, another secondary material is used: potassium gold cyanide, a microcrystalline powder that is made from dissolving gold via electrolysis in potassium cyanide. The synthesizing of potassium gold cyanide and potassium cyanide both use electrical and thermal energy (Rajagopal 165). These major materials used for gold-plated jewelry each require large amounts of energy to produce and are from finite sources. Even before the manufacturing process starts, the acquisition and processing of raw materials into secondary materials for manufacturing embeds an unsustainable amount of energy into a single piece of gold-plated jewelry.
Once the materials reach the factory, processing begins. The manufacturing process of gold-plated brass jewelry requires constant energy thanks to the abundance of chemical and physical processing that needs to occur. In order to turn alpha-beta brass and potassium gold cyanide into jewelry, factories begin with modeling a piece of jewelry in mind. Most companies use 3D rendering to create a piece of jewelry before creating a silicone mold for it from the 3D printed version. The silicone molds are injected with microcrystalline wax to create wax “trees” which are wax jewelry models with more than one piece of jewelry attached to the “tree” in order to bulk production easily (Wang et al. 684). The wax models are placed in “investment” , a casting material that surrounds the wax tree creating a mold that will not melt when molten metal is poured into it. What will melt is the wax, as the molten alpha-beta brass fills the cavity where the wax was, creating a brass version of the wax tree. Each individual jewelry piece is cut from the tree and subjected to cleaning and polishing before the electroplating process (Qingdao TopN). Machines used at each step of processing embed energy into the materials, the machines transforming fossil fuels into thermal and kinetic energy to mold, melt and cast. The brass pieces of jewelry are next electroplated with a few layers of brass first, for the gold plate to adhere better. To prepare for plating, the brass jewelry is submerged into a metal salt (a combination of positively charged gold ions and an acid) bath. The bath used in jewelry manufacturing is typically a low PH acid cyanide bath, made from gold cyanide, citric acid, cobalt, potassium hydroxide and water heated to 180F. Thermal energy converted from fossil fuels is used for melting the wax, brass, and heating the cyanide bath; the gold cyanide bath alone uses 1.07GJ/1g of solution per year (Mazzeo, Holcombe 115). An electrical current is applied to the solution, depositing positively charged gold ions onto the negatively charged brass jewelry. The thickness of the gold plate is dependent on how long a piece of jewelry is submerged, but most gold plate is extremely thin, with an average thickness of only 0.5 microns (Wilkinson 76) which is why it is so cheap to produce and why the gold wears off easily. Although the gold will come off in probably less than a year, the energy used annually in the electroplating process alone is an estimated 1.75 x10^5 kwhs (Mazzeo, Holcombe 113) of electrical energy used to provide an electrical current in the bath. Metabolic energy of factory workers tasked with polishing, operating and maintaining machinery is also an energy expenditure that contributes to the embodied energy of gold-plated jewelry. By using heavily processed industrial materials, gold-plated brass jewelry factories expend high amounts of energy to chemically and physically process the materials into a finished jewelry product. One gold plating factory in China alleges their factory produces over six million pieces of jewelry annually (Qingdao TopN). Fossil fuels are the main source of energy for the individual machines and power plants that electrify factories such as that one. Due to the energy density of fossil fuels, manufacturing gold jewelry embeds incredible amounts of energy within.
Common gold-plated jewelry that can be found in any American boutique was likely manufactured overseas, usually in China. Transportation and distribution of gold-jewelry is fueled by gasoline and oil, the main sources of energy for all modes of transportation and distribution. In order to be shipped, the jewelry is cleaned and packaged into plastic wrappers, and into cardboard boxes. From there, the jewelry can go anywhere, being distributed by car, truck and freighters. Just one average size freighter (8,000-9,000 TEU) requires at least 225 tons of crude oil fuel a day (Jean Paul Rodrigue et al. 134), and using crude oils’ energy density of 44MJ/kg, means that any jewelry being shipped on an average freighter for a day, has accrued 8981 gigajoules of energy. Once the jewelry lands at its port of destination, gasoline powered vehicles distribute the quantities to brick-and-mortar stores, or to back end distribution centers for web sales. Gasoline and crude oil are both finite fossil fuel sources that create GHG emissions, all in the name of distributing consumer product. Since most gold-plated jewelry is made outside of the United States, more energy is needed in the life cycle of gold-plated jewelry because it has to travel in order to be bought by a consumer. Locally made products do not require the crude oil energy of a shipping freighter, but jewelry manufactured overseas in large quantities does, accruing embedded energy along the way.
Arguably the only way to use gold-plated jewelry is to wear it. Consumers wear gold-plated jewelry for its expensive appearance, cheap price tag, and low maintenance. It’s only maintenance involves keeping the jewelry away from water, and gently cleaning occasionally, all of which use minimal metabolic energy from the consumer. It’s vital to keep the jewelry away from water and clean it gently, because the gold plate can be easily stripped away to reveal the brass underneath (“Make Your Gold Plated Jewelry Last Longer”). Brass can corrode and oxidize once freed from the gold plate and exposed to air and moisture, causing the wearer of the gold-plated jewelry to be displeased. The common complaint being “it turned my skin green”, a result of copper within the alloy oxidizing green onto the skin. The only way to reuse the gold-plated jewelry after the plate has worn off is to get the jewelry replated, an expense often worth more than the jewelry itself. There are few ways you can recycle your gold-plate jewelry and that’s only if there is still gold to be salvaged from it (Lenson). This is the inherent problem with gold-plated jewelry, it cannot be easily maintained, reused or recycled by the average consumer, destining almost every single piece of cheaply gold-plated jewelry to the landfill. Not to mention the energy needed to transport the jewelry from trash can to landfill is dependent on the gasoline powered garbage trucks and the distance to the nearest waste management facility. All the energy that went into making a single piece of jewelry is never recuperated into the life cycle either through reuse or recycling, wasting the materials and energy used throughout the entire cycle.
Gold-plated jewelry uses valuable materials and energy that are non-renewable and contributes to the unsustainable consumption and extraction of our finite material and fuel sources. When gold, copper, and zinc are being mined just to go back into the ground, or be rubbed off into oblivion, there must be a fundamental change in the mass manufacture of cheap gold-plated fashion jewelry to lessen the embodied energy it requires. The chemical process of electroplating cheap alloys uses electrical, chemical, and thermal energy, three types of energy to apply a temporary artifice of gold but there are no measures in place to recapture that energy. Consumers and manufacturers need to rethink accessible and cheap jewelry and figure out how to turn the linear life cycle of gold-plated jewelry, circular.
Works Cited
Callcut, Vin. “Innovations: Introduction to Brasses (Part II).” Www.copper.org, Copper Development Association Inc, Jan. 2000, www.copper.org/publications/newsletters/innovations/2000/01/brasses02.html. Accessed 20 Oct. 2021.
“Copper Mining and Processing: Processing Copper Ores.” Superfund Research Center, University of Arizona, 13 July 2020, superfund.arizona.edu/resources/learning-modules-english/copper-mining-and-processing/processing-copper-ores#:~:text=Copper%20mining%20is%20usually%20performed. Accessed 2 Nov. 2021.
“Http://Topn.dstopnn.com/.” Topn.dstopnn.com, Qingdao TopN Import and Export CO LTD, 2006, topn.dstopnn.com/. Accessed 4 Nov. 2021.
Jean-Paul Rodrigue, et al. The Geography of Transport Systems. 5th ed., London, Routledge, 2020, p. Chapter 4.1: Fuel Consumption by Containership Size and Speed.
Lei, Tianyang, et al. “Adaptive CO2 Emissions Mitigation Strategies of Global Oil Refineries in All Age Groups.” One Earth, vol. 4, no. 8, 20 Aug. 2021, pp. 1114–1126, www.cell.com/one-earth/fulltext/S2590-3322(21)00410-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2590332221004103%3Fshowall%3Dtrue, 10.1016/j.oneear.2021.07.009. Accessed 22 Oct. 2021.
Lenson, Barry. “Are Gold Plated and Gold Filled Items Worth Recycling.” Reclaim, Recycle, and Sell Your Precious Metal Scrap, Specialty Metals, 30 Nov. 2020, www.specialtymetals.com/blog/2020/11/30/thinly-gold-plated-and-gold-filled-items-once-inexpensive-are-worth-investing-in-today. Accessed 5 Nov. 2021.
“Make Your Gold Plated Jewelry Last Longer.” Gold Plating Services, 26 Apr. 2018, www.goldplating.com/blogs/news/make-your-gold-plated-jewelry-last-longer. Accessed 5 Nov. 2021.
Mazzeo, Daniel Angelo, and W. D. Holocombe. Energy Conservation Study of the Plating and Surface Finishing Industry : Phase I. Atlanta, Engineering Experiment Station, Georgia Institute Of Technology, 1978.
Palou-Rivera, I, and Wang, M Q. Updated estimation of energy efficiencies of U.S. petroleum refineries.. United States: N. p., 2010. Web. doi:10.2172/1009352.
Rajagopal, Indira, and S R Rajagopalan. “Electrochemical Preparation of Potassium Gold Cyanide.” Bulletin of Materials Science, vol. 6, no. 2, May 1984, pp. 165–175, 10.1007/bf02743894. Accessed 23 Oct. 2021.
Sykes, Clare, et al. Zero Emission Copper Mine of the Future. The Warren Centre for Advanced Engineering, 2020.
Wang, Jiayi, et al. “Design and Topology Optimization of 3D-Printed Wax Patterns for Rapid Investment Casting.” Procedia Manufacturing, vol. 34, 2019, pp. 683–694, 10.1016/j.promfg.2019.06.224. Accessed 22 Act. 2021.
Allison Bennett
DES 040A
Professor Cogdell
2 December 2021
The Waste and Emissions of Gold-Plated Jewelry
Gold-plated brass jewelry is a fashion staple that often ends up sitting and rusting in many people’s closets. This cheaply made accessory is a hot commodity for people looking to save a couple of bucks while still being in “style”. The short lifespan and “disposable” nature of the product lead to many of these accessories providing sizeable amounts of waste in landfills. After weeks of wear, gold-plated jewelry will oxidize leaving a pesky green tint on any skin it touches. That ultimately turns them into “trash” and leads the mass-produced item to be thrown away after a short consumption period. Not only is the product itself unsustainable as it does not have a long wear period, but there is also an equal amount of waste and emissions that come as a result of the collection of raw materials and manufacturing process. Gold-plated brass jewelry is a cheaply made unsustainable mass-marketed product that releases overwhelming amounts of waste and chemical emissions from cradle to grave. Providing waste and emissions during the acquisition of the raw materials, the manufacturing process, packaging and distribution, use, maintenance, recycling, and waste management; the benefits of gold-plated brass jewelry are not worth the environmental impacts and waste byproducts that come along with it.
Gold-plated brass jewelry produces waste through each step of its life cycle, starting from the very beginning with the extraction and acquisition of raw materials for manufacturing. A majority of the waste that is created in this step of the process is from mining the raw materials. In order to produce gold-plated brass jewelry, the materials: gold, beta brass, microcrystalline wax, and gold cyanide are required. Each of these materials is acquired differently supplying their own arrays of wastes and emissions. Gold is collected through a mining process, where there are emissions of Carbon Dioxide and Mercury into the atmosphere. It is estimated that between 640 mg to 1350mg of mercury are released into the environment in the gold mining process, a significant amount of that being released into the atmosphere (Telmer K.H., Veiga M.M.). Mining usually requires diesel-powered machinery to collect materials out of the ground, which release carbon monoxide (CO), carbon dioxide(CO2), nitric oxide (NO), nitrogen dioxide (NO2), sulfur dioxide (SO2), and various other hydrocarbons (Bugarski). An assessment of the environmental impacts on metal acquisition shows that the intense use of fossil fuel combustion for the operation of machinery used for mining is directly related to climate change and fossil fuel depletion impacts. Data shows, “69% of greenhouse gases and 67% of oil equivalent are emitted and depleted by primary gold and silver production”(Thammaraksa et al.). In a report from EarthWorks and MiningWatch Canada, gold and other metal mining companies dump roughly 180 million tons of toxic waste into rivers, lakes, and oceans each year (Hyeyeun and Schroder). Then there is the collection of “alpha-beta brass”. Which is a duplex brass that is an alloy created by roughly 40% zinc and roughly 60% copper (Callcut). The process of mining the two metals is fairly similar to the mining of the gold, as they use diesel machinery as well for acquisition. The alpha-beta brass is a secondary material used to create a cheaper alternative to sole metals (Callcut). Microcrystalline wax, which is used for mold casting in the manufacturing step of the production process, is a product of petroleum refinement (Lei et al. 1115). There are significant amounts of mercury in wastewater and gaseous emissions as a result of petroleum refining. Exact numbers of mercury concentrations in crude oil, or petroleum, cannot be determined in entirety at the moment due to the lack of documentation from crude oil companies (Willhem). It is clear that there are significant emissions resulting from the material acquisition phase of the life cycle, even before any product has been made.
During the manufacturing process, a majority of the emissions produced are a result of the fossil fuels used to power the required machinery for production. In order to begin production, the alloy is shipped to the plating factory. Releasing carbon dioxide into the atmosphere as it is shipped (Thammaraksa et al.). Once the alpha-beta brass has arrived at the factory it is cast into a microcrystalline wax mold to produce the shape of the base jewelry. The molten metal is poured into these “wax trees” in the casting process. In the melting and casting stage of production, produces residues from ingot dressing as well as wastewater from graining (Corti). This wastewater often contains cyanide and dissolved metals which are hazardous to humans and the environment (“Wastewater Produced in Jewelry Manufacturing”). Most jewelry factories have an attached sewage system where this wastewater “sludge” is disposed of, where it sits for years (“Wastewater Produced in Jewelry Manufacturing”). The next step in the jewelry manufacturing process is electroplating. The molded jewelry is placed into a metal salt and water combination for electroplating. This “magic liquid” is made from gold cyanide, citric acid, cobalt, potassium hydroxide, and water (Adhoum et al.). “The electroplating process creates a considerable amount of toxic wastewater containing heavy metals, which requires treatment” (Adhoum et al.). This is due to the fact that the electroplating process requires the jewelry to sit in a new bath of liquid every time they are plated. Meaning that there is a lot of water and metal particulates being wasted in the process. Due to the high metal content, the wastewater must be treated before being released into the environment. Treatment of electroplating wastewater has several forms, precipitation, adsorption, ion exchange, and reverse osmosis. The most commonly used form of wastewater treatment is precipitation(Adhoum et al.). This process is based on chemical coagulation, a process where they add lime to raise the pH and add aluminum salt to remove the colloidal matter. Although the process has proven quite successful in treating the wastewater and making it safer for disposal, the precipitation method has proven to produce secondary emissions by the added chemical substances (Adhoum et al.). There are significant amounts of product waste and emissions throughout the manufacturing stage in the gold-plated jewelry product life cycle, but it doesn’t end there. In order to market and sell the jewelry to the consumer, the product must first be packaged and shipped which adds to the overall waste produced.
The third stage of the life cycle for gold-plated brass jewelry is its distribution. It is normally shipped internationally from China and other trading capitals of production. Where it is packaged in unnecessary amounts of single-use plastics. Packaging is an integral part of the distribution of jewelry, and basically all other shipped products. Corporations spend over 60 million dollars a year on packaging products for sale. It is a widespread thought by manufacturers that packaging should “impress the consumer’s eyes” or more specifically, it must be aesthetically appealing (Rocha et al.). The jewelry industry primarily packages its products in rigid paperboard and plastic boxes (Chen and Ebrahimpour). In general, the packaging used for jewelry is hard to dispose of. Materials like fabric, plastic, and metal have slow degradation periods. They, along with the jewelry will eventually end up sitting in a landfill for many years. Then on top of that, the products require extra protection, normally paper, as well as an additional plastic casing to protect the product from the elements during the shipping process (Rocha et al.). All of which end up in the trash can as soon as the consumer receives their product. Once the individual products are packaged, they are placed into even more packaging suitable for the shipping process. Commonly, this is cardboard. Cardboard comes along with its own set of wastes and emissions. Cardboard for one is heavy to transport resulting in higher vehicle emissions and fuel costs than the plastic alternative (Plank). If not recycled, cardboard is a biodegradable product and releases methane into the environment (Plank). Once it is time to send the jewelry to its distributor, the products must be shipped out. Global shipment can be done by ship or plane. In a study, ships emitted 932 tonnes of CO2 in 2015 from total shipping internationally and domestically (Olmer, Naya, et al.). It is absurd how much waste is produced in order to deliver the gold-plated brass jewelry to the consumer.
The sole use for gold-plated jewelry is to wear it, consumers will buy these cheap accessories and wear them till they inevitably fail, throwing them away to sit in landfills. On average a gold-plated necklace has a lifetime of roughly 2 years before it begins to tarnish (Zafar). After the two-year mark, or even less, the jewelry begins to tarnish and rust. Once it has rusted the product is virtually trash as it will just oxidize and turn the wearer’s skin green. There is a way to clean the jewelry and maintain it for longer with a Jewelry Steam Machine, but often for the cheap price of the plated jewelry and the amount of effort that is required to get jewelry cleaned, the majority of the product ends up being tossed away(Zafar). A small number of metal scraps from the production and use of gold-plated jewelry are able to be smelted and cleaned for reuse creating an array of chemical emissions in the process. In some cases, scraps and other gold-plated brass jewelry wastes can be collected and refined for technical and economic reasons (Corti). The process of these scraps and wastes being re-alloyed to be resued in the jewelry making process produce some pollution releases toxic emissions. The chemicals in these emissions are like those released in the wastewater from the manufacturing step in the lifecycle. Cyanide and toxic metallic runoff are the main pollutants (Corti). It’s safe to say a minority of gold-plated brass jewelry that is used by a consumer will end up being sent into the proper channels to be recycled and refined for material reuse. It was hard to find specifics on how much jewelry is wasted and sent into landfills yearly, but there is some data on fast fashion as a whole. In recent years, more products, including plated jewelry, are purchased and wasted than ever before. Roughly 57% of these purchased fashion products will end up sitting in landfills across the globe (“Fashion and Waste: An Uneasy Relationship.”).
Through this project, it became apparent just how much waste can be produced by one tiny product. The time, materials, energy, and waste produced to create a small piece of jewelry is remarkable, and not at all worth it. Toxic waste is released into bodies of water and harmful pollutants are released into the atmosphere, all for one small necklace to be worn for 2 weeks and tossed away. As well as cardboard, plastic, and paper waste from packaging are all put in to send a product with a 2-year life span for aesthetic purposes. This whole life cycle design project really puts into perspective the power and implications correlated to consumerism. If there’s a market, people will buy it…if people buy it, production will create double the amount of waste from it. Next time you think about buying that $20 necklace at Kohls, think about what you are supporting and how you could put your money into something more sustainable and renewable.
Works Cited
Adhoum, N, et al. “Treatment of Electroplating Wastewater Containing Cu2 , Zn2 and Cr(VI) by Electrocoagulation.” Journal of Hazardous Materials, vol. 112, no. 3, 2004, pp. 207–213., https://doi.org/10.1016/j.jhazmat.2004.04.018.
Bugarski, Aleksandar D. et al. Controlling Exposure to Diesel Emissions in Underground
Mines. Englewood, Colorado: Published by Society for Mining, Metallurgy, and Exploration, Inc., 2012. Print.
Chen, Shaw K., and Maling Ebrahimpour. “Demand Forecasting for a Jewelry Packaging Company: Pattern Identification and Assessment.” International Journal of Production Economics, vol. 22, no. 3, 1991, pp. 203–209., https://doi.org/10.1016/0925-5273(91)90096-c.
Corti, Christopher. “Recovery and Refining of Gold Jewellery Scraps and Wastes.” May 2002.
“Fashion and Waste: An Uneasy Relationship.” Common Objective, Common Objective, 8 June 2018, https://www.commonobjective.co/article/fashion-and-waste-an-uneasy-relationship.
“Introduction to Brasses (Part II).” Innovations: Introduction to Brasses (Part II), https://www.copper.org/publications/newsletters/innovations/2000/01/brasses02.html.
Jeon, Hyeyeun, and Jackson Schroeder. “How Sustainable Is Your Jewelry?” The University Network, 18 June 2021, https://www.tun.com/blog/how-sustainable-is-your-jewelry/.
Lei, Tianyang, et al. “Adaptive CO2 Emissions Mitigation Strategies of Global Oil Refineries
in All Age Groups.” One Earth, vol. 4, no. 8, 20 Aug. 2021, pp. 1114–1126,
www.cell.com/one-earth/fulltext/S2590-3322(21)00410-3?_returnURL=https%3A%2
F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2590332221004103%3Fshowall%3Dtrue, 10.1016/j.oneear.2021.07.009. Accessed 22 Oct. 2021.
Olmer, Naya, et al. “Greenhouse Gas Emissions From Global Shipping, 2013–2015.” Https://Theicct.org/, Oct. 2017.
Plank, Melanie. “How Sustainable Is Paper And Cardboard Packaging?” Common Objective, 24 Jan. 2020, https://www.commonobjective.co/article/how-sustainable-is-paper-and-cardboard-packaging.
Rocha , Silvia, et al. “Jewelry Packages: Some Interpolations on Design and Form.” Advances in Human Factors, Business Management, Training and Education, Springer, 2016, pp. 327–336.
Telmer K.H., Veiga M.M. (2009) World emissions of mercury from artisanal and small scale
gold mining. In: Mason R., Pirrone N. (eds) Mercury Fate and Transport in the Global
Atmosphere. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-93958-2_6
Thammaraksa, Chonlawan, et al. “Corporate Environmental Assessment of a Large Jewelry Company: From a Life Cycle Assessment to Green Industry.” Journal of Cleaner Production, vol. 164, 2017, pp. 485–494., https://doi.org/10.1016/j.jclepro.2017.06.220.
“Wastewater Produced in Jewelry Manufacturing.” Dtsc.ca.gov, https://dtsc.ca.gov/wastewater-produced-in-jewelry-manufacturing-fact-sheet/.
Wilhelm, S. Mark. “Estimate of Mercury Emissions to the Atmosphere from Petroleum.” Environmental Science & Technology, vol. 35, no. 24, 2001, pp. 4704–4710., https://doi.org/10.1021/es001804h.
Zafar, Sadaf. How Long Does Gold Plated Jewelry Last After Proper Care?, FoxyNature, 30 Apr. 2021, https://foxynature.com/how-long-does-gold-plated-jewelry-last-249.
Chelsea Maeglin DES 40A
December 1, 2021
Gold Plated Jewelry Life Cycle Assessment: Raw Materials
Gold plated jewelry has become an increasingly popular product in recent years as it has provided a broader range of consumers with an opportunity to engage in the jewelry market. By using a less expensive base metal and plating it with a very thin coating of gold, producers are able to create pieces that appear to be made of gold for a fraction of the price of using completely precious metals. Because of this, gold plated jewelry is offered at a much lower price range than regular gold pieces (Simple & Dainty). While this more affordable product initially appears to be a wonderful solution to the cost barrier associated with jewelry for consumers in a lower socioeconomic bracket, gold plated jewelry has actually been a detrimental development for many reasons. At the core of the problematic nature of gold plated jewelry as a product are two components: a low threshold for wear and no long term maintenance solutions, a combination that leads to an inevitably short life for these beautiful pieces that are ultimately destined to end in a landfill. Not only is Gold Plated jewelry a wasteful and environmentally damaging product in and of itself, but the processes that are associated with the extraction and acquisition of raw materials to create such jewelry have detrimental effects themselves.
In each of the seven stages of its life cycle, the process of creating gold plated jewelry requires different raw materials. The first step in the life cycle and process of creating gold plated jewelry is the extraction of the metals that are used to create the jewelry. As the name Gold plated implies, these pieces contain gold. Ironically, however, they are only approximately 0.05% or less gold by weight (Simple & Dainty). The rest of the jewelry is composed of a base metal, most commonly copper or the cheaper alternative, beta-brass, which is an alloy of Copper and Zinc with high levels of zinc. These metals create the form and structure of the jewelry upon which the gold is later plated. (Gold Plating Services) Because the jewelry is made using multiple metals, the metal extraction process for gold plated jewelry has two notable components: the mining of base metals such as copper and Zinc, which is used to make beta-brass, and the mining of gold for plating. While this is only the first step, it has by far the most detrimental impacts on the environment. Before the materials have even made it to the factories for production, this jewelry has already contributed to industrial environmental repercussions.
Mining of the base metals, the primary materials used in the production of this jewelry, has notable negative repercussions. There are large amounts of waste associated with mining as whatever material that is being sought, in this case the ore that is mined, usually makes up only a very small piece of the material excavated from the earth (Dudka and Adriano 590). Aside from the large amounts of waste generally implicit in mining, there are known additional waste products associated with copper and zinc mining and processing, in particular. Both copper and zinc are mined as sulphides which, when exposed to water or air, create sulphuric acid which turns ground water into acid water (Sengupta 1). This acid water impacts the ecosystems in which it occurs as wildlife is disrupted by changes in the ground water (Sengupta 1). In addition to the adverse impacts of copper and zinc mining, it should be noted that as Stanislaw Dudka and Domy C. Adriano stated in their 1997 review of the environmental impacts of metal ore mining and processing, “Cu smelting emits approximately 0.11 Mg of S per Mg of Cu produced in the USA. Zinc and Pb smelters release large quantities of Cd and Pb into the environment. Metal smelting and refining produce gaseous (CO2, SO2, NOx, etc.) and particulate matter emissions, sewage waters, and solid wastes.” (590) Before the gold plated jewelry has even begun to be made, the extraction and processing of the base metals alone have already caused massive environmental impacts.
While most metal mines have a disproportionate amount of waste for the amount of product they are able to procure, gold mines are especially disruptive as they are usually open pit mines. In order to extract the gold from the rock that is mined, either cyanide leaching or mercury is used (Fernandez and Klimas 2) Notable impacts of gold mining include: excessive earth displacement, mercury and cyanide in waste which can cause erosion and other damage to ecosystems, and a reduction in air quality due to elemental mercury that is released into the air (Smithsonian). According to research done by the Smithsonian, “...the Environmental Protection Agency has reported that 40 percent of watershed headwaters in the western United States have been contaminated by mining operations.” While cyanide is not a catastrophic waste product because of its short half life, the sheer amount of cyanide contamination due to the size of the gold industry is problematic. Mercury, on the other hand, is a lethal chemical and a correlation has been found between living near mining sites and higher levels of mercury in individuals’ bodies (Fernandez and Klimas 2). While the aforementioned impacts of gold mining are the most prevalent in the conversation about its environmental consequences, it is important to be conscious of the high levels of carbon dioxide emissions associated with the mining processes as well (Fernandez and Klimas 2).
Once the metals have been extracted from the earth as ore and then processed into a usable state, the next step in the life cycle of gold plated jewelry is the molding process in which the jewelry starts to take shape. At this stage in the process, the notable raw materials that get introduced are silicone and microcrystalline wax, both of which are used for the casting process. The silicone is used as the material that makes up the molds into which the base metals are poured. Silicone is a synthetic polymer that is derived from silica and made by passing the extracted silicon through hydrocarbons (Silicone Engineering). The microcrystalline wax that gets used in the molding process is a petroleum based or hydrocarbon wax. While waxes with high levels of purity aren’t known to be particularly harmful, “most industrial waxes have a lower level of purity and their polyaromatic hydrocarbon (PAH) content—mainly originating from the paraffin extraction process—can cause irritation to the skin and eyes” (Suaria, Giuseppe, et al 4). While there is documentation of the potential harmful impacts associated with exposure to these impure waxes, there is limited information available regarding the specific impacts of the production of these waxes themselves.
After the base jewelry has been cast using the crystalline wax and silicone molds, the next step in the process is the gold plating process from which this notorious product derives its name. The base jewelry is gold plated in a bath made from potassium gold cyanide and aqua regia, the only material introduced at this stage, which is a combination of nitric and hydrochloric acids (Grot 1). The gold is plated onto the jewelry using electrical currents that bond the gold, which carries a positive charge, to the base metal which carries a negative charge. While there are potentially adverse effects of the acquisition of aqua regia, they are relatively insignificant in the context of the gold plated jewelry life cycle as they pale in comparison to the scale of the environmental damage due to the acquisition of the metals.
Once the jewelry has been plated, the penultimate step of the life cycle is packaging and distribution. Most of the jewelry will find itself in some mechanism of packaging that involves a thick paper product or cardboard. While cardboard is recyclable, making it one of the most sustainable elements of the process, it is still made from paper and therefore derived from trees. No matter the perspective taken, there are adverse effects that result from the deforestation and use of trees associated with paper and cardboard production. Another element frequently used in the packaging process is plastic. While there is a range of plastics used to package materials such as jewelry, many of the options are single use plastics which have widely recognized and negative impacts on the environment. Whether the packaging is made sustainably or not, it is important to note that “Excessive use of packaging materials is creating many environmental problems.” (Varun and Nautiyal)
After the Jewelry is delivered and worn, it will eventually begin to degrade. Because of the thinness of the gold plating and the chemical reactions of the base metals, gold plated jewelry is notorious for tarnishing quickly and producing undesirable green reactions with the skin (Gold Plating Services). Gold plated jewelry is not recyclable so after all of the energy expended, materials mined and created, and labor, there is little the consumer can do to care for their jewelry in a way that would meaningfully extend its lifetime. The only remotely viable option for extending the life of a gold plated piece is getting it re-plated, a process that often costs more than the jewelry did in the first place and uses even more gold.
While lack of information regarding the exact impacts of acquiring some of the raw materials make it difficult to do a comprehensive life cycle assessment on gold plated jewelry, it is evident that the processes associated with the acquisition of raw materials needed to produce gold plated jewelry are damaging to the environment and furthermore, the short life times of these pieces do not justify such detrimental impacts.Unfortunately, gold plated jewelry is a large contributor to environmentally damaging practices and not only is the product itself unsustainable due to its short lifetime and susceptibility to wear but it also is unsustainable in its creation from the moment the first raw materials are acquired.
Works Cited
Dudka, Stanislaw, and Domy C. Adriano. “Environmental Impacts of Metal Ore Mining and Processing: A Review.” Journal of Environmental Quality, vol. 26, no. 3, 1997, pp. 590–602., https://doi.org/10.2134/jeq1997.00472425002600030003x.
Fernandez, Jessica and Klimas, Christie (2019) "A Life Cycle Assessment of Jewelry," DePaul Discoveries: Vol. 8 : Iss. 1 , Article 6. https://via.library.depaul.edu/depaul-disc/vol8/iss1/6
“Gold Plated vs. Gold Filled Jewelry.” Gold Plating Services, https://www.goldplating.com/blogs/news/gold-plated-vs-gold-filled-jewelry.
Grot, Walther. “Applications.” Fluorinated Ionomers /, 2011, pp. 81–156., https://doi.org/10.1016/B978-1-4377-4457-6.10005-6.
Magazine, Smithsonian. “The Environmental Disaster That Is the Gold Industry.” Smithsonian.com, Smithsonian Institution, 14 Feb. 2014, https://www.smithsonianmag.com/science-nature/environmental-disaster-gold-industry-180949762/.
Norgate, T.e., et al. “Assessing the Environmental Impact of Metal Production Processes.” Journal of Cleaner Production, vol. 15, no. 8-9, 2007, pp. 838–848., https://doi.org/10.1016/j.jclepro.2006.06.018.
Northey, S., et al. “Using Sustainability Reporting to Assess the Environmental Footprint of Copper Mining.” Journal of Cleaner Production, vol. 40, 2013, pp. 118–128., https://doi.org/10.1016/j.jclepro.2012.09.027.
Salles, Fernanda Junqueira, et al. “The Environmental Impact of Informal and Home Productive Arrangement in the Jewelry and Fashion Jewelry Chain on Sanitary Sewer System.” Environmental Science and Pollution Research, vol. 25, no. 11, 1 Feb. 2018, pp. 10701–10713, 10.1007/s11356-018-1357-z. Accessed 23 Oct. 2021.
Sengupta, Mritunjoy. “Environmental Impacts of Metal Ore Mining and Processing.” Environmental Impacts of Mining, 2021, pp. 137–142., https://doi.org/10.1201/9781003164012-5.
Suaria, Giuseppe, et al. “The Occurrence of Paraffin and Other Petroleum Waxes in the Marine Environment: A Review of the Current Legislative Framework and Shipping Operational Practices.” Frontiers, Frontiers, 1 Jan. 1AD, https://www.frontiersin.org/articles/10.3389/fmars.2018.00094/full.
Thammaraksa, Chonlawan, et al. “Corporate Environmental Assessment of a Large Jewelry Company: From a Life Cycle Assessment to Green Industry.” Journal of Cleaner Production, vol. 164, Oct. 2017, pp. 485–494, 10.1016/j.jclepro.2017.06.220.
“Types of Gold Jewelry Explained: Plated, Vermeil, Filled, & Solid Gold.” Simple & Dainty, https://simpleanddainty.com/blogs/guides/types-of-gold-jewelry.
Varun, Sharma A., Nautiyal H. (2016) Environmental Impacts of Packaging Materials. In: Muthu S. (eds) Environmental Footprints of Packaging. Environmental Footprints and Eco-design of Products and Processes. Springer, Singapore. https://doi.org/10.1007/978-981-287-913-4_5
“Where Does Silicone Rubber Come from? Learn with Us Today.” Silicone Engineering, 10 Sept. 2021, https://silicone.co.uk/news/where-does-silicone-rubber-come-from/.