Design Life-Cycle
assess.design.(don't)consume
Grace Moon
DES 040A – Fall 2016
Professor Cogdell
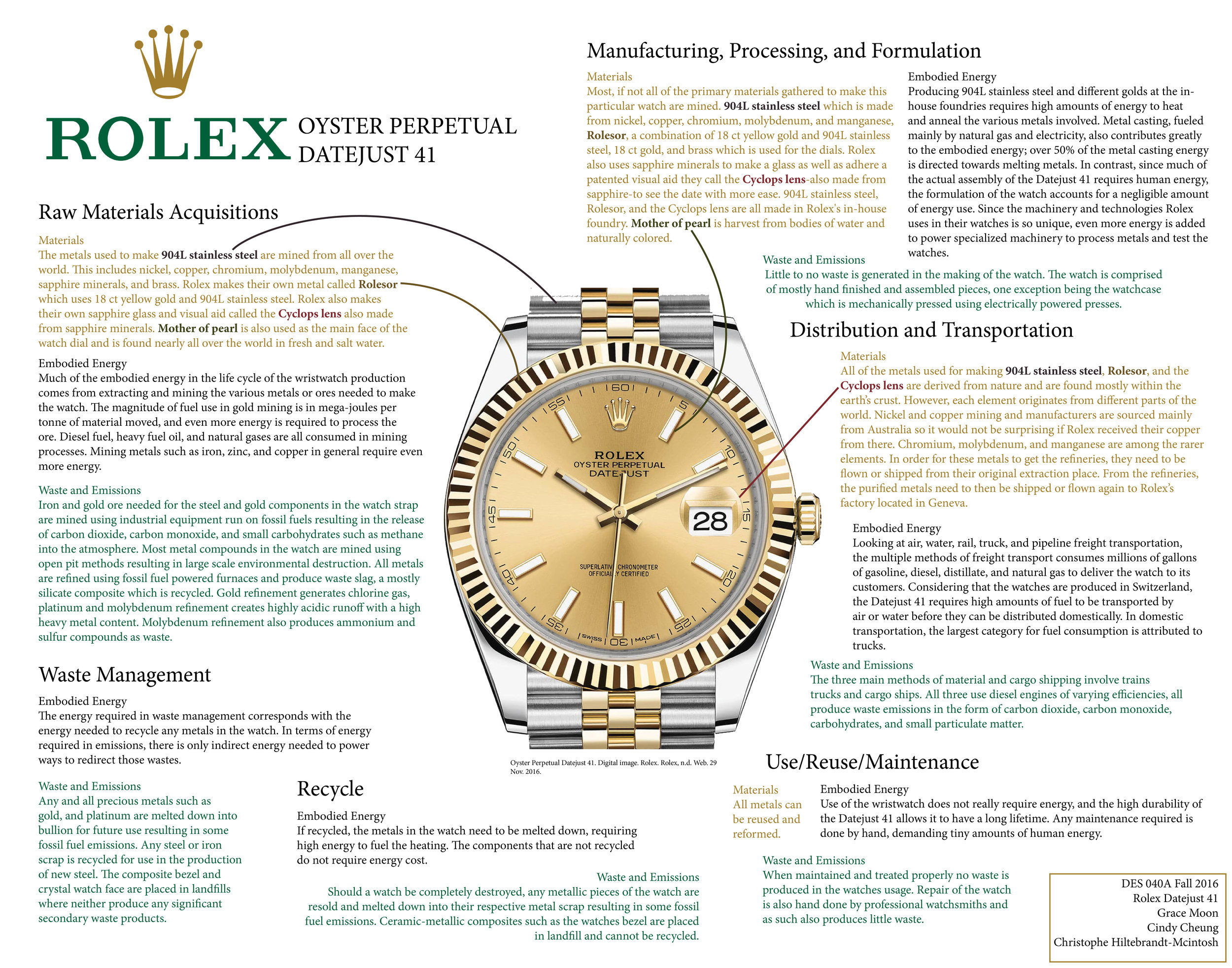
Raw Materials: Rolex Datejust Watch 41
Most people do not stop to think about where companies get their materials for their product, let alone how it is made. The question of where these materials come from often gets overlooked and is something we fail to think about unless specifically asked to do. In order to answer this question, we need to look at three main aspects of a product’s full life cycle, the raw materials, the embodied energy, and the waste and emissions that the product uses and undergoes while being made. The particular product our group chose to examine is the Rolex Oyster Perpetual, Datejust 41 watch. As students studying energy and material through design we want to analyze and examine the specific materials used in the Rolex Datejust 41 and determine if the cost of gathering and producing material for this luxury watch is worth the environmental damage it causes against the earth.
Since this paper’s focus is on the raw materials we will take a closer look at what is being used for each part of the watch. The cohesive metallic harmony of the watch makes it hard for us to imagine being several different pieces of metal making it even easier to forget what materials are exactly being used. In order to understand the damage the materials can have on the environment, we first need to know where they are being produced and how they all get to Rolex’s headquarters. Although this information was very difficult to find, after much research, calling Rolex, and making educated assumptions, I was able to put together a sum of my findings.
Firstly, Rolex uses 904L stainless steel for most of their watches. In order to make 904L steel, they need to first get a hold of the raw primary materials needed to make it. Many of the specific origins of the raw primary materials Rolex uses are unknown; even when I tried calling Rolex, the lady who answered at the retail store did not know. What is only known is that they have a small closely-knit group of suppliers that send them steel (Adams, 10 Things…Rolex Watches). However, based on outside research, I was able to assume that the raw metals and materials are gathered from different areas of the world through typical mining techniques like surface and sub-surface (underground) mining, depending on what is being extracted (Hudson, Fox, and Plumlee, 17-19). They also tell us that after they gather their primary raw materials, they make all of their secondary raw materials in their in-house foundry like their 904L stainless steel, patented Rolesor, Cyclopes lens, and mother of pearl (Rolex, Materials). Based off of this information, we are able to further analyze the materials and better understand the energy and waste emissions that go into making this luxury watch.
Rolex’s in-house foundry is arguably what sets them apart from all other high-end luxury watches. It is here that they make all of their secondary raw materials that enable them to control the quality of their watches. The main component of the Rolex Datejust 41 and most of their other watches is 904L stainless steel. This makes up the largest and most visible areas of the watch, including the bracelet, links of the bracelet, inner case, and dial parts. For this reason, it is very important to learn where the metals used to make 904L come from. Most watch companies use a type of stainless steel called 316F and although this type of metal also has high corrosion resistant properties with good polishing, it is less hard and less corrosion resistant than 904L stainless steel due to its reduced concentration levels of nickel, chromium, and molybdenum (AZoM, Stainless Steel - Grade 316F). Rolex’s patented version of 904L stainless steel contains nickel, chromium, copper, molybdenum, and manganese, mostly consisting of nickel and chromium (AZoM, Stainless Steel-Grade 904L). Depending on the location of the mines, the ways in which each of these elements is extracted will differ and will use different amounts of energy and processes (Murdoch, How Gold…Ground).
Rolex’s 904L stainless steel plays a huge part of the materials component of the watch because it is used as a primary and secondary raw material as it is also combined with yellow gold to make Rolex’s patented Rolesor. All of the metals used for making 904L stainless steel are derived from nature and are found mostly in the earth’s crust. It is important it keep in mind that because each of these metals come from different parts of the world, the damage the Datejust 41 can have on the environment will be more than initially expected. Nickel is mined in over 23 countries but not all 23 countries have refineries, therefore, the raw metals would need to be transported to nickel refineries. The largest copper mining and manufacturers are sourced from Australia so it would not be surprising if Rolex received copper from there (NPI, Copper & Compounds). Chromium is mined in South Africa, India, Turkey, Kazakhstan, and Albania (MEC, Chromium). Manganese is not found as a free metal in nature but is found as a mineral mixed with other minerals such as silicates, oxides, and carbonates (NPI, Manganese & Compounds). Because of this, the refining process for manganese can be difficult which adds to the cost of buying the materials for the watch being made. In order for these metals to get the refineries, they need to be flown or shipped from their original extraction place. From there, they get transported to the refineries and the purified metals need to then be shipped or flown to Rolex’s factory located in Geneva. Nickel and copper are among the more common elements and would most likely cost less economically and environmentally than copper chromium, molybdenum, and manganese (WebElements, Geological Information). The amount of transportation in exporting and importing costs not only more money but also more in energy and fuel due to the number of steps it takes to extract, refine, process, and transport these metals. The economic cost of actually mining, refining, processing, and transporting these materials is unknown but it can be assumed that Rolex spends a large amount of money getting them which is costing more in energy expenditure to obtain these resources because they are flown to and from different countries multiple times.
Other patents that Rolex has is one for a metal they named Rolesor. Rolesor is their creation of yellow gold and 904L stainless steel combined. This patented metal makes up the bezel, winding crown, center bracelet links, and outer case of the watch (Rolex, Datejust 41). The effects of mining as a whole are very damaging to the environment resulting in “long-lasting effects due to the due to the mechanisms of metal cycling in tropical forest ecosystems” (Salomons, 5). In order for Rolex to acquire all of their metals, mining must take place. Unfortunately, mining produces a lot of waste and emissions in more than one way, damaging the environment atmosphere, ecology, waterways, and landscapes (Salomons, 6).
The Rolex Datejust 41 also has a patented magnifying Cyclops lens over the date to help with visibility (Rolex, Datejust 41). The Cyclops lens is an extra piece of sapphire crystal glued to the larger crystal above the date window (Becker, Rolex Cylops). Rolex gets their sapphire crystals as well as watch hands from outside suppliers and produce the Cyclops lens in-house through special heat treatments (Adams, Inside Rolex…). Sapphire must be mechanically separated from the clay and gravel and can also be broken up by a high-pressure water jet (AZoM, Sapphires-Processing…). Although there were no results that could be found for the exact process Rolex does to laminate their glass, Apple Inc. does a similar process. The method includes lapping and polishing a first side of a sapphire sheet and adhering the sapphire sheet to a glass sheet. It also includes lapping and polishing a second side of the sapphire sheet to chemically strengthening the glass sheet (WO 2013134159 A2, 2012).
Rolex also uses a material called mother of pearl which “is the common name for iridescent nacre, a blend of minerals secreted by oysters and other mollusks that is deposited in their shells to protect and coat them from parasites and foreign objects” (Wickell, Mother of Pearl). The mother of pearl can vary in colors, ranging from pink, white, black, or yellow depending on what part of the shell it is extracted from (Rolex, Materials). The mother of pearl can be found in most places all over the world in fresh and salt water (GemSelect, Mother of Pearl). All of the dials at Rolex are used for the dial face of the watch. Just like the raw metals, the mother of pearl would need to be transported several times to factories to be refined and extracted to be used as a final product.
Other models of the Oyster Perpetual use 950 platinum, Everose gold, and pure 904L stainless steel, all made in-house with their own foundry. 950 platinum is an alloy consisting mainly of ruthenium, copper, cobalt, iridium, rhodium, or palladium (Brilliance, Platinum). Everose gold is Rolex’s own creation of 18 ct pink gold made in their foundry. The primary raw metals and minerals needed for these watches are all found from outside mining sources all over the world. Rolex does not disclose the names of the specific companies who supply them. Because of this, we need to be extra attentive to the ways that they use their energy and manage their waste emissions in obtaining these materials in order to determine if the benefit of owning one of these luxury watches is really worth the amount of environmental damage it has. One good thing about these metals is that they are all recyclable; the metals can all be reheated and melted into new shapes and forms. With the left over metals Rolex has, they use it to produce the smaller gears for the inside of the watch (Rolex). Because this is such a high quality watch, once a finished watch is sold, many times it does not break or scratch, therefore not thrown away and kept for years and even generations.
In conclusion, we can see that there are many different parts of the Rolex Datejust 41 than when initially looked at. From the mining processes to the in-house foundry and factories that Rolex has, we can see just how many materials go into making a single watch. Although it was difficult to find certain information about where Rolex actually obtains their materials, overall I feel successful in being able to gather the main raw primary materials that they used and where they generally come from. Based on assumptions from other sources related to metals and mining and further research, we can make an educated guess on how Rolex acquires their materials. The amount of energy needed and the environmental effects of gathering these materials may not be worth it in the long run.
Works Cited
Adams, Ariel. "10 Things To Know About How Rolex Makes Watches." ABlogtoWatch. A Blog to Watch, 24 Nov. 2013. Web. 29 Nov. 2016.
Adams, Ariel. "Inside Rolex: Understanding the World's Most Impressive Watch Maker." Forbes. Forbes Magazine, 5 Dec. 2013. Web. 29 Nov. 2016.
AZoM. "Sapphires - Processing, Grading, Characteristics, Heat Treatment and Marketing of Sapphires." AZoM.com. H.C. Starck Ceramics GmbH, 16 Aug. 2003. Web. 29 Nov. 2016.
AZoM, Written By. "Stainless Steel - Grade 904L (UNS N08904)." AZoM.com. N.p., 7 Nov. 2001. Web. 29 Nov. 2016.
AZoM, Written By. "Stainless Steel Grade 316F (UNS S31620)." AZoM.com. N.p., 4 Mar. 2013. Web. 29 Nov. 2016.
Becker, Matthew. "What's a Rolex Cyclops?" Beckertime, LLC. N.p., 10 Mar. 2014. Web. 29 Nov. 2016.
"Chromium | Minerals Education Coalition." Minerals Education Coalition. Black and Veatch Corporation, n.d. Web. 30 Nov. 2016.
Fox, Frederick D., Geoffrey S. Plumlee, and Travis L. Hudson. Metal, Mining and the Environment. Alexandria, VA: American Geological Institute, 1999. Print.
"Materials." Rolex. Rolex, n.d. Web. 29 Nov. 2016.
Metallurgy Corp. "Gold Smelting & Refining Process." Mineral Processing & Extractive Metallurgy. N.p., 03 Nov. 2016. Web. 29 Nov. 2016.
"Mother of Pearl Gemstone Information." Mother of Pearl Gemstone Information. N.p., 19 May 2015. Web. 29 Nov. 2016.
Murdoch, Julian. "How Gold Gets Out Of The Ground." How Gold Gets Out Of The Ground | ETF.com. N.p., 18 May 2009. Web. 29 Nov. 2016.
Oyster Perpetual Datejust 41. Digital image. Rolex. Rolex, n.d. Web. 29 Nov. 2016.
Prest, Christopher D. Https://www.google.com/patents/WO2013134159A2?cl=en. Patent WO 2013134159 A2. 12 Sept. 2013. Print.
"Rolex Datejust 41 Watch: Yellow Rolesor - Combination of 904L Steel and 18 Ct Yellow Gold - 126333." Rolex. Rolex, n.d. Web. 29 Nov. 2016.
Salomons, W. "Environmental Impact of Metals Derived from Mining Activities: Processes, Predictions, Prevention." Journal of Geochemical Exploration 52.1-2 (1995): 5-23. Google Scholar. Web. 29 Nov. 2016.
Wickell, Carly. "What Is Mother of Pearl?" About.com Style. N.p., 06 Mar. 2016. Web. 29 Nov. 2016.
Cindy Cheung
Professor Christina Cogdell / DES 40A Fall 2016
Rolex Datejust 41 Watch Embodied Energy
A Rolex watch: without a doubt, a coveted luxury item that many view as a status symbol. Little do Rolex consumers and enthusiasts know just how much effort and energy it takes to bring them the pricey, classic watch. The company started with Hans Wilsdorf, who first founded Rolex S.A. in London of 1905, eventually moving it to Geneva in 1919 (“History of Rolex”). Due to their extremely high standard for their wristwatches, Rolex became a highly vertically integrated company, with most of the materials and assembly processes achieved in-house. Their 19 wristwatch types include the Rolex Datejust 41, a self-winding “classic watch of reference” first introduced in 1945 and distinguished by its 41mm size, striking steel and yellow gold Jubilee bracelet, fluted bezel, and a window by the dial displaying the date (“Datejust 41”). By analyzing the energy used in the life cycle of a Rolex Datejust 41, it becomes apparent the mindlessly high amount of embodied energy Rolex focuses towards processing, assembling, and delivering such a specialized, luxury wristwatch to the customer.
Due to Rolex’s desire to produce unique and durable watches, much of the energy of the wristwatch production comes from processing the various materials needed to make the watches. Before the materials used to make the watch are even processed, raw materials such as iron, molybdenum, nickel, gold, copper, iron, manganese, sapphire, ruthenium, and mother of pearl, need to be extracted. According to the Barrick Gold Corporation, which had a clear report of its mining processes, the amount of energy needed in mining gold was 34.4 megajoules (106 joules) per tonne of material moved, while the amount of energy needed in processing was 138.1 MJ per tonne of ore processed in 2015 (Advancing. Together.). The 2015 Responsibility Report from Barrick Gold Corporation also included the fuel use of their mining process. The breakdown of energy used includes: 16.9 gigajoules (109 joules) of energy in diesel fuel, 14.2 GJ in heavy fuel oil, 10.7 GJ in natural gas, and 1.5 GJ in other fuel uses (Advancing. Together.). As one of the largest gold mining companies in the world, these numbers provide a good estimate of how much energy gold mining and processing takes, even if it is not the company Rolex gold is supplied by. To give a reference of how much energy mining metals (such as iron, gold, zinc, and copper) takes, the Mining Industry Energy Bandwidth Study estimates that 552.1 Tbtu (trillion british thermal unit = 1,055,870 GJ) is consumed by the mining industry annually (BCS, Incorporated). Only after the materials have been mined can the real making of the watch materials begin.
Most of these raw materials undergo processing at the Rolex Geneva Plan-les-Ouates site, where the framework of the watch is developed and machined (“Rolex-made in Switzerland”). Some of the defining features of the Datejust 41 are the Jubilee, “a supple and comfortable five-piece link metal bracelet,” and Rolesor, which is their patented combination of 904L stainless steel and 18ct gold (“Datejust 41”). The Plan-les-Ouates site is the location of the in-house foundry, which is where Rolex’s specialized gold and steel compounds are created (“Rolex-made in Switzerland”). 904L, a stainless steel composed mostly of iron, chromium, nickel, and molybdenum, needs to be heated to 2,000 to 2,200º F as well as fully annealed immediately afterward (“Stainless Steel 904L”). In addition to the mining energy needed to produce this steel, it also requires energy to process this specific steel designed for high corrosion resistance (“Stainless Steel 904L”) In a report for the U.S. Department of Energy, it is predicted that the minimum values to produce steel is about 8,150 MJ/tonne for integrated production and 1,600 MJ/tonne for scrap-based production (Fruehan et al.). Since this grade of stainless steel has a unique process and requires other metals, it is safe to assume that 904L steel production surpasses the report’s minimum energy prediction. Metal casting, a process in which liquid metal is poured into molds to form shapes, also requires much energy to power. The Advanced Melting Technologies report estimates that 60% of energy use in metal casting is fueled by natural gas and 27% of energy use is fueled by electricity (Naranjo et. al.). It also reports that melting the metal is the most highly energy intensive aspect of metal casting, taking up 55% of the energy costs, while the rest of the energy cost is distributed across heat treatment, post casting, coremaking, moldmaking, and other processes (Naranjo et.al.). Although I was not able to find the specific metal quantities used to make each Datejust 41, the sheer amount of mining and metal-processing energy required reveals a high embodied energy cost.
Once the foundation of the wristwatch – the bracelet and case – is fabricated, the smaller components such as the movements, dials, and gems are assembled mostly through handwork. Three sites contribute most to the assembly of the Datejust 41, each focusing on a different aspect of the production. The Geneva Chêne-Bourg site includes dial manufacturing and gemology, the Bienne site handles the production of movements, and the Geneva Acacias site (the headquarters) finishes and tests the final product (“Rolex-made in Switzerland”).
At the Chêne-Bourg site, at least 100 workers at a time produce, paint, and set dials, apply numerals, and hand-set gems, according to Benjamin Clymer, founder of HODINKEE (an established online wristwatch magazine) who was invited to tour the four sites of Rolex. The dials and dial markers are produced using precious metals, so the workers go through many processes before the perfect dial components are achieved (Clymer). Since the work here is mainly completed through human labor, I decided to look at human work capacity to contrast the other embodied energy amounts required in the rest of the watch production processes. According to John R. Wilson and Nigel Corlett in Evaluation of Human Work, light energetic work, has average energy expenditures of up to 249 watts and up to 14 kilojoules per minute (Wilson and Corlett 439). More specifically, a summation of average energy expenditures according to work movements and postures provided by Wilson and Corlett gives a more accurate output: basal metabolic rate corresponds to 85 W, sitting corresponds to 20 W, back bent forwards corresponds to 15 W, and dynamic work with hands and fingers corresponds to up to 65 W of energy expenditure (Wilson and Corlett 440). If that is all added up, sitting with back bent forwards and using both arms to do dynamic work with hands and fingers comes down to an average energy expenditure of up to 250 watts of work, equivalent to 250 joules per second of energy. Even by a comparison of the units used, there is no competition between processing energy versus human labor energy.
At the Bienne site, where over 2,000 people engage in the machining and hand-assembling of unique Rolex movements, operators and watchmakers work “with the precision of the order of a few microns … to meet the accuracy criteria of the Swiss Official Chronometer Testing Institute (COSC)” (“Rolex-made in Switzerland”). The Datejust 41 features a calibre 3235 movement, a self-winding mechanical movement which uses technology with 14 patents, improving “precision, power reserve, resistance to shocks and magnetic fields, ease of use, and reliability,” according to their e-brochure on the Datejust 41 (Rolex). To achieve this technology, gold nuts, a self-winding motor invented in 1931 by Rolex, high-performance lubricants developed in-house, a patented blue Parachrom hairspring, and tools only accessed by Rolex-certified watchmakers are all used (“Perpetual Movements”). The blue Parachrom hairspring seems to be made of a paramagnetic alloy of niobium, zirconium, and oxygen, according to a blog post citing a Rolex communication from August 12, 2006 (Ficklin). It was difficult to find much information on the blue Parachrom hairspring, although seemingly so crucial to the precision of Rolex watches, however, many forums and blog posts that I searched through seemed to agree on this information. Yet another piece of technology that has multiple materials that must be first extracted and processed before it is then formed in-house and hand-assembled into the watch, it only adds in more energy to the Datejust 41’s life cycle.
Finally, at the world headquarters, the Rolex site at Acacias, the final assembly and quality control testing is executed (“Rolex-made in Switzerland”). After the Datejust 41 is fully assembled, it is put through a string of intense tests to ensure waterproofness and precision. Pressurized tanks are used to simulate the guaranteed depth of waterproofness for each watch, which adds in more energy to the equation since they need to be powered (Clymer). Then, components of the wristwatches are examined again, a 24-hour accuracy test is employed, bracelets are hand-fitted, and COSC tags are applied all by workers at the Acacias site (Clymer). This extensive checking and testing process before it is finally taken to destination markets takes up a lot of time and accumulated human energy to ensure the Rolex standard of perfection.
It can be assumed then that distribution of these luxury watches must take up a significant amount of energy, as it is a Swiss watch company that has official retailers located all over the world. I was not able to find much on the distribution channels of Rolex watches, but came to the assumption that they must be shipped first to strategic flagship stores in various countries, which then must be distributed among the official retailers across the country. I was not able to find the fuel consumption specifically for Switzerland’s air transportation, but I found that the U.S. consumed 5,988.4 million gallons of fuel in 2015 for international air carriers and 10,741.2 million gallons of fuel in 2015 for domestic air carriers (Airline Fuel Cost and Consumption). After the watches are shipped to various countries, we must also consider the energy use involved in transporting the product around the country. According to the report Freight Facts and Figures 2006, 68% of fuel consumption is attributed to trucks, 16% is attributed to water transport, and 8% each is attributed to Class I rail and to pipeline transport (Mallette et. al.). By highway, 173,750 million gallons of gasoline, diesel, and other fuels was consumed in 2004, and for rail in freight service, 4,059 million gallons of distillate or diesel fuel was consumed in 2004 (Mallette et. al.). These high levels of fuel consumption undoubtedly contribute towards greenhouse gas emissions, mainly through emitting nitrogen oxides (Mallette et. al.) Freight transportations account for 27% of U.S. nitrogen oxide emissions, with trucks contributing the greatest amounts (Mallette et. al.). Due to the high number of trucks on the road combined with the other modes of international and domestic freight transport, fuel consumption plays a significant role in the embodied energy aspect of the Datejust 41 watch life cycle.
The last aspect of the Rolex Datejust 41 wristwatch life cycle pertains more to the embodied energy Rolex requires as a whole highly vertically-integrated production. As noted previously through the patented materials and processes described, the components of a Rolex are extremely specialized, which implies unique machinery needed to process and test the watches. Even more so than other manufacturing companies, then, Rolex requires high amounts of electricity use. According to the U.S. Energy Information Administration, machine drives accounts for about half of the manufacturing sector’s delivered electricity use (Otis). Energy use in machine drives includes electricity consumed by machinery, transportation equipment, fabricated metal, nonmetal minerals, chemicals, primary metals, petroleum and coal, and others that are less relevant to Rolex (Otis). In addition to specialized machinery, it takes a copious amount of energy to power the four Rolex sites, each of which have up to 11 stories (some of which are underground), implying that even more lighting technology that usual is needed (Clymer). The Bienne site occupies 92,000 square meters, according to Clymer, so I will use this estimate as an approximate building space volume for the rest of the sites as well. A report on commercial building energy usage attributes 80,000 Btu per square foot of total energy used (2012 Commercial Buildings Energy Consumption Survey: Energy Usage Summary). Using 0.093 square meters per square foot as a conversion, we can estimate that the Bienne site occupies 990,279.76 square feet. This means that the Bienne site consumes 79.2 billion Btu of total energy. Using that as the standard for the other sites, a total of 316.8 billion Btu is used to power the entire system of Rolex production. On the plus side, since they are so durable and timeless, I would assume that most Rolex customers keep these expensive wristwatches for a long time or at least within the family, so the energy required to use, maintain, and dispose of the watches is minimal. Perhaps the actually assembling of the Datejust 41 wristwatch, itself, is not a highly energy intensive process, since much of it is hand-made; however, the overall energy use in maintaining the sites and machines used to process the components of the wristwatch adds up to an incredible amount.
Through analyses of each step of the Rolex Datejust 41 wristwatch’s life cycle, it can be concluded that most of the embodied energy required does not come from the assembly of each watch; rather, the intense energy consumption originates from the processes that surround that production. The highest concentrations of energy use are contained in raw material extraction, metal casting, distribution through freight transport, and Rolex site maintenance, while the hand-done assembly of each watch requires almost negligible human energy. Rolex, as a highly vertically integrated company, somewhat blindly works towards improvements on their products without considering the environmental effects of high energy consumption in order to bring a product that achieves its high standards for wristwatch perfection.
Works Cited
Advancing. Together.: 2015 Responsibility Report. Rep. Barrick Gold Corporation, 2015. Web. 28 Nov. 2016.
Airline Fuel Cost and Consumption. Rep. U.S. Department of Transportation. Bureau of Transportation Statistics, 28 Nov. 2016. Web. 28 Nov. 2016.
BCS, Incorporated. Mining Industry Energy Bandwidth Study. Rep. U.S. Department of Energy, June 2007. Web. 28 Nov. 2016.
Clymer, Benjamin. "Inside The Manufacture: Going Where Few Have Gone Before – Inside All Four Rolex Manufacturing Facilities." HODINKEE. HODINKEE, 16 Mar. 2015. Web. 14 Nov. 2016.
“Datejust 41.” Rolex. N.p., n.d. Web. 27 Nov. 2016.
"Dynamic Work Assessment." Evaluation of Human Work. Ed. John R. Wilson and Nigel Corlett. 3rd ed. Boca Raton: CRC, 2005. 438-39. Print.
Ficklin, Jordan. "Rolex Parachrom Hairspring." Blog post. Tick Talk. Wordpress, 7 May 2008. Web. 28 Nov. 2016.
Fruehan, R.J., O. Fortini, H.W. Paxton, and R. Brindle. Theoretical Minimum Energies To Produce Steel. Rep. Pittsburgh: Carnegie Mellon U, 2000. Web. 25 Oct. 2016.
“History of Rolex.” Rolex. N.p., n.d. Web. 27 Nov. 2016.
Naranjo, Robert D., Ji-Yea Kwon, Rajita Majumdar, and William T. Choate. Advanced Melting Technologies: Energy Saving Concepts and Opportunities for the Metal Casting Industry. Rep. Maryland: BCS, Incorporated, 2005. Print.
"Perpetual Movements." Rolex. N.p., n.d. Web. 26 Oct. 2016.
Otis, Paul. "Electricity use by machine drives varies significantly by manufacturing industry" U.S. Energy Information Administration. U.S. Energy Information Administration, 13 Oct. 2013. Web. 25 Oct. 2016.
Mallette, William, Rolf Schmitt, and Joanne Sedor. Freight Facts and Figures 2006. Rep. no. FHWA-HOP-07-033. N.p.: U.S. Department of Transportation Federal Highway Administration Office of Freight Management and Operations, 2006. 42. Nov. 2006. Web. 27 Nov. 2016.
Rolex. Datejust 41. N.p.: Rolex, n.d. Rolex. Rolex. Web. 28 Nov. 2016.
"Rolex-made in Switzerland." Rolex. N.p., n.d. Web. 26 Oct. 2016.
“Stainless Steel 904L.” Metal Suppliers Online, n.d. Web. 25 Oct. 2016.
2012 Commercial Buildings Energy Consumption Survey: Energy Usage Summary. Rep. U.S. Department of Energy. U.S. Energy Information Administration, 18 Mar. 2016. Web. 28 Nov. 2016.
Christophe Hiltebrandt-McIntosh
Des 40A-Fall 2016 A01
The Rolex Datejust 41: Waste and Emissions
Close your eyes and think about luxury. What do you see? Fast cars, tall skyscrapers, gilded hallways, soaring arches, no? Perhaps this isn’t what you see when you think of luxury but if I told you to think of luxurious watches, the brand Rolex would certainly be on it. But as with all luxurious things, the price we have to pay for them is one of their defining characteristics. Now if I asked you the price of a Rolex watch, in this day and age you could easily search and not only tell me its price in US dollars but you could conceivably tell me every price for every watch ever sold by Rolex in every currency used on earth, but that’s not the price I’m concerned with. I’m concerned with the environmental price of a Rolex watch. Take the Datejust 41 sold by Rolex, what is the environmental price tag that accompanies this watch, and how environmentally friendly is it? Specifically, this paper will explore just how environmentally friendly the Datejust 41 is through a close examination of the waste and emissions that go into the life cycle of Rolex’s Datejust 41 watch.
We begin our lifecycle of the Datejust 41 at the ground level, its natural components. Now I could rattle off each and every material used in the watch, like gold, platinum, 904L steel, molybdenum ect. Since many of the Datejust’s natural components are metallic and as such undergo very similar ore extraction and refinement methods I will highlight only a few, namely gold, platinum, molybdenum and 904L steel as they highlight the breadth of each varying ore refinement process and the wastes produced. Gold (Au) is strange as far as metals go as it doesn’t react with many other elements on the periodic table and as such is often found in nature as a chemically pure substance. This means that little if any chemical treatment is necessary to isolate gold from its other earthen counterparts. One exception to this is the Miller process in which chlorine gas is passed over molten gold along with a small addition of borax and silicates, this combination reacts with any trace impurities in the molten gold and is run off as waste, the good news is that this process is used only for the most minute amounts of impurities and any and all chlorine gas, a lead cause of acid rain, is recycled back into the process. platinum, however, is a different story.
To understand the waste produced in the platinum refinement process it is first important to understand how rare platinum is. Platinum comprises of about 3.7*10^-6% of the earth’s crust, opposed to the far more common iron, which settles at around 6.3%. Due to platinum’s rarity as well as well as its chemical nature, no ores exist where platinum comprises a majority of the chemical structure. As such, platinum refinement is often done alongside other precious metal separation for only small samples where tests have shown a high concentration of platinum or other precious metals such as iridium, palladium, and ruthenium. The process first starts by grinding the samples into a fine powder then separating the powder based on precious metal content using a technique called froth flotation where the finely ground ores are mixed into a slurry, and placed in a container while air is bubbled from beneath and the foam from the slurry is separated. The result of this flotation separation is a matte with a high concentration of pgm’s, also known as platinum group metals. This matte is then reground and smelted using reverberatory furnaces. These furnaces are coal powered and as such produce all the waste typically assumed when burning coal, carbon dioxide, carbon monoxide, small particulate matter and carbohydrates. The smelting process also produces slag, which is the term given to the concentration of impurities that are formed due to the smelting process. Fortunately, slag is often recycled for later use in concrete, asphalt, and other masonry, only an approximate 2% of slag is disposed of in landfills. Once smelted, what remains after the slag is run off is then placed in a mixture of nitric and hydrochloric acids (aka agua regia). This in turn dissolves many of the platinum group metals which are later dried to form varying types of salt. These salts are then converted into pure platinum using formic acid. Now as you can probably tell by the chemicals and processes used in the refinement of platinum that the waste from platinum refineries is highly acidic and contains trace amount of heavy metals, the types of metals which were capable of dissolving in the acid but incapable of being reduced into pure metal by the formic acid. As a result, waste runoff from platinum refineries is incredibly hazardous to life as pH of platinum runoff settles at around 1, highly acidic, and contains trace elements of iron, nickel, and zinc at concentrations of <10ppm. Fortunately, these metals in low concentrations can prove harmless to aquatic life. however, the high acidity of waste runoff is deadly as most forms of marine life as they rely on a stable pH balance to survive, and in the case of coral reefs, require pH to be above a certain point in order to even form. So while platinum and other precious metal may be considered valuable their impact on the environment through the acidification of soil and water must be considered.
Molybdenum, similar to platinum undergoes extensive chemical treatment in order to refine the material into its pure form. Depending on its initial state and concentration molybdenum ores can undergo a multitude of different chemical processes that each invariably produce their own unique chemical waste. That being said I will only focus on the high concentration molybdenum ore, molybdenite. Molybdenite can undergo one of two different processes, one using two separate chemical reduction cycles, using hydrochloric acid and hydrogen gas. The other process using a crystallization process in turn of the hydrochloric reduction reaction. In either case the molybdenite is first roasted and leeched using ammonium hydroxide to form molybdenate. This molybdenate solution is then purified chemically to reduce sulfur content in the solution; creating waste in the form of sulfide compounds. From here on out the two processes split. In one this purified molybdenate solution is evaporated and re-crystallized to form ammonium-paramolybdate crystals, in the other, hydrochloric acid is introduced to precipitate ammonium-paramolybdate. Once again these two processes rejoin at this point onward. The advantage of using hydrochloric acid to form the ammonium-paramolybdate is it can be done in bulk and far more quickly than the evaporation and recrystallization process, however both processes have downsides as using hydrochloric acid produces acidic waste and the recrystallization process requires energy to evaporate the water left in solution, resulting in the usage of fossil fuels. From here the ammonium paramolydate crystals are reduced using hydrogen gas to form pure molybdenum metal and ammonium hydroxide waste. The molybdenum is then reheated to form ingots which consequently results in more waste being produced through the use of energy to recast the molybdenum. As you can tell, similarly to platinum, molybdenum produces quite a few acidic waste products along with a few sulfurous compounds. Both processes rely heavily on the use of energy to heat, melt and reheat solutions and metals in order to reduce or recombine them, resulting in yet more emissions waste produced by these furnaces. So once more we see the presence of acid waste in a metallic refinement process, this time accompanied by sulfurous waste and ammonium compounds. But surely 904L steel is better, right?
To look at the environmental impact of 904L steel we must start at its origins in the types of ore it can be found in. 904L begins its life as one of four possible iron ores: Hematite, Magnetite, Limonite, and Siderite. For our purposes we will focus on hematite and magnetite as these are the ores primarily used in stainless steel production. Both hematite and magnetite are ferric oxides, meaning they chemically comprise of solely iron and oxygen. As such the refinement and processing of these ores are largely similar. The refinement process, begins by crushing the ore into finer pieces, this stage yields little to no emissions on the part of the ore itself. This ore sand is then fed into a blast furnace along with limestone, a purifying agent, and coke, the fuel/carbon source, to form what is known as ‘pig iron’. Waste is inherently abundant in this process as the limestone is used to bind with the oxygen and other impurities in the ore which floats to the top of the blast furnace and is removed. Treatment for Iron slag recycling is identical to that of platinum refinement slag. From here, the pig iron is blasted in an oxygen furnace with recycled steel scrap to make steel. Once more coal is used to power the furnace and as such produces the usual waste associated with coal burning. Once the steel has been produced the mixture then has trace amounts of Nickel and molybdenum added to create the highly corrosive resistant 904L steel. What’s good about this is that the oxygen furnaces that finalize the production of steel produces very little waste in and of itself, usually in the form of carbon monoxide, trace sulfur compounds and oxide dusts most of which are then recycled back into the process. The downside being that amongst the massive energy use required to fuel the different furnaces, both rely on the same source of fuel, coke.
So what is coke? The material has cropped up quite a bit, from furnaces to roasting, coke seems to be everywhere in the industrial world, and with good cause. But to answer simply, coke is essentially petrified coal. The process to make coke is very similar to that of charcoal, you burn coal in a very low oxygen environment to remove any non-combustible materials from the coal, this is what makes it such a good fuel and carbon source for iron smelting. However, in its use and in its manufacturing it produces quite a few waste emissions. To list a few: Methane (119lbs/ton), Carbon Monoxide (48.2lbs/ton), Carbon Dioxide (20.9lbs/ton), Hydrogen sulfide (6.6 lbs/ton), and Hydrogen cyanide (2.1lbs/ton). This emission waste is only from the production of coke, not its actual usage. You may have noticed that many if not all of these chemicals released in cokes production are either greenhouse gasses, toxic to human beings, or both. Considering this, also note that in cokes usage it produces additional carbon dioxide/monoxide. So on the whole not very environmentally friendly to say the least. But unfortunately, coke is a necessary part in the production of 904L steel as smelted iron needs a source of concentrated carbon from which to draw upon so when the iron cools it locks these carbon molecules in its lattice creating the tough and durable steel that goes into the Datejust 41. The use of coke in furnaces who do not need coke for its carbon source has been the matter of environmental debate for some time. Alternative methods for iron production could include electrical furnaces with pure carbon in the form of graphite or other recycled materials being used as a carbon additive substitute. But this solution assumes that the electricity used to heat said furnaces is supplied completely by renewable resources, which frankly isn’t the case. Coke while unclean, and a definite source of waste in the production of the Datejust 41, is a necessity in producing quality iron and steel and is extremely cost effective from the perspective of molybdenum and platinum refineries.
Now that the waste inherent in the production of the raw materials that go into the Datejust 41 have been revealed, it is time we looked at how these materials were transported. In transportation there are two types, that which travels over land, and over water. There is also air transport but for commercial shipping purposes we can ignore it as air travel becomes exceedingly expensive for manufacturers, especially when shipping heavy metallic components in bulk. This is demonstrated by the transport of goods in the US; air transport takes only .3% of the market. This leaves us with land and water transportation. Over land the preferred method is the train, taking up 39.5% of the market, followed by trucks at 28.6% of the market. The remaining 31.6% of the market is reserved by varying types of pipeline transportation, unsuitable for transporting metallic goods. So how are trains and trucks powered? Contrary to what most people would think, the majority of trucks and trains are diesel powered, albeit trains are up to four times more fuel efficient than trucks, capable of moving one ton of freight at a rate of 470mi/gal of fuel. But what waste gasses are produced in diesel engines? Similar to burning coke, diesel engines produce waste in the form of carbon monoxide, carbon dioxide, hydrocarbons and other small particulate matter. But what about water transportation? As it turns out, cargo ships use diesel engines too! So how do we find out how much waste due to transportation goes into the Datejust 41 watch? First we’ll have to make a few assumptions, we are going to assume that the steel and other components come from south America, as south America has some of the largest mines for the natural resources in the Datejust 41 in the world. Net we have to assume that the raw materials are processed somewhere in the US, and then shipped over to Switzerland by cargo ship, as air travel can be easily ruled out. So we take the distance of those two stretches (~4544mi, and ~4975mi respectively), get the MpG for both types of transport, an average of 470mi/gal for freight and cargo ship at 8.01*10^-4mi/gal of diesel. Adding in conversion factors for carbon dioxide, we get that for the trip an approximate 6.21*10^6 gal of diesel used and an astounding total of 1.37*10^8 lbs of carbon dioxide produced. Now naturally these shipping methods do not solely ship Datejust 41 watches and as such aren’t representative of the carbon dioxide produced through the transport of one single watch. This number also doesn’t take into account the amount of carbon dioxide produced per pound of cargo for each shipping method nor does it estimate any of the other waste products like carbon dioxide or hydrocarbon waste produced in the transportation. As such this is a pretty rough estimate of the waste produced in the transportation of the Datejust 41 watch’s raw materials to Switzerland. With transportation and raw material manufacturing and refining out of the way, what waste comes into play once the materials arrive at Rolex’s factories?
Truthfully, very little waste is created in the production of these watches as they arrive in Switzerland. This is largely due because Rolex makes large use of skilled human labor. Granted, machines are used to tool larger pieces and Rolex has a specific machine designed to press watch cases from 904L steel but all dials, flywheels, springs, and the wristband are all finished, polished and assembled by human hands and as such any and all machine assistance and waste is essentially negligible. No doubt Rolex’s use of human labor is one of the reasons the brand Rolex is synonymous with quality, luxury, and expense.
But what happens when you break that luxury? How long does a Rolex watch truly last? Typically, when a Rolex is broken it is brought to a watchmaker or a dealer where, depending on the severity of the damage done to the watch the broken pieces will either be repaired or replaced. While replaced parts are no longer part of the watch, their components are still very valuable due to the expensive nature of their metallic materials and as such will often be recycled. The one exception of this is the patented cerachrom bezel which is a metallic-ceramic composite, where once broken is dumped in the landfill. Despite this the cerachrom bezel of the Datejust 41 is designed specifically to last lifetimes. But if we focus on the metal parts, environmentally speaking metal is beautiful. Once it has been purified and cast, whether corroded or broken you can always melt it back down and cast it once more into new pieces. Gold is an excellent example of this, with its low melting point and easily malleable surface people have been recycling and reusing gold to break down and remake products since golds discovery. Even in today’s market, recycled gold makes up for more than a third of all gold we see today. The one downside of all this is the energy needed to reheat and re-melt pieces. While on a small scale many jewelers use electric furnaces, many industrial metal recycling plants use coal to fuel their furnaces. That’s not saying that electricity is all that clean either. Using the US as an example, as of 2015 33% of the US’s electricity was generated using coal. So despite being a very renewable resource, metal as a whole still produces waste long after its initial lifespan has ended in an endless cycle of cast, break, reheat, and recast. As far as the lifespan of the Datejust 41? Accounts vary but most agree that if taken care of and professionally cleaned every five years, the lifespan is theoretically infinite.
So that’s the life cycle of the Datejust 41. While not altogether “cradle to cradle” it certainly fits the description of ‘cradle to ad-infinitum’. the environmental costs of the transportation and production that goes into the Datejust are high, no doubt about it. But if examined over the watch’s theoretically infinite lifespan can it be said that the watch is environmentally friendly? That ultimately depends on what your definition of environmentally friendly is. If something is environmentally friendly when it produces no waste or emissions, then no the Datejust 41 is extremely environmentally costly. From the environmental damage caused by open pit mining, or the acidification of oceans due to air pollution, mining and refining metals has never been, nor do I believe ever will be, an entirely waste free process. But if you look at the Datejust 41 watch from a comparative point of view, it can easily be said to be environmentally friendly. Rolex watches don’t have off gassing effects as they are recycled, they aren’t inherently toxic like batteries or other electronics, and don’t pollute our oceans like plastics do. Now while the idea of a garbage patch in the ocean comprised entirely of Rolex watches may seem ludicrous, perhaps the very fact that we find it a ludicrous notion speaks for itself, for who on earth would throw away a Rolex watch? Perhaps this is the true face of luxury, a product that no one would throw away, a product that instead of becoming waste, lives on through our continued efforts to reuse the old and recycle the broken. Just imagine if we treated the whole world as a luxury too precious to spare.
Works Cited
"904L Stainless Steel." Tampa Bay Steel. TampaBaySteel Co., n.d. Web. 24 Oct. 2016.
"About Platinum." About Platinum. Goldandsilvermines.com, n.d. Web. 27 Nov. 2016. <http://www.goldandsilvermines.com/platinum.htm>.
Andrews, Anthony, and Richard K. Lattanzio. Petroleum Coke: Industry and Environmental Issues. Rep. no. 7-5700. Congressional Research Service, 29 Oct. 2013. Web. 27 Nov. 2016. <http://www.nam.org/CRSreport/>.
Association, IMOA International Molybdenum. "Molybdenum Chemical LCI." Description of Chemical LCI. N.p., 2006. Web. 24 Oct. 2016.
Azevedo, Ligia B., An M. De Schryver, A. Jan Hendriks, and Mark A. J. Huijbregts. "Calcifying Species Sensitivity Distributions for Ocean Acidification." Environmental Science & Technology Environ. Sci. Technol. 49.3 (2015): 1495-500. Web. <http://pubs.acs.org/doi/full/10.1021/es505485m>.
AZoM, Written By. "Stainless Steel - Grade 904L (UNS N08904)." AZoM.com. AZO Materials, 30 Apr. 2014. Web. 24 Oct. 2016.
B, Duke James, Greene Ernest W, Hunter Joseph L, and Minerals & Chem Philipp Corp. "Patent US2990958 - Froth Flotation Method." Google Books. N.p., 4 July 1968. Web. 24 Oct. 2016.
Boyd, Claude E., and Laurence Massaut. "Risks Associated with the Use of Chemicals in Pond Aquaculture." Aquacultural Engineering 20.2 (1999): 113-32. Web.
"Common Uses for Slag." Common Uses for Slag | National Slag Association. National Slag Association, n.d. Web. 27 Nov. 2016. <http://www.nationalslag.org/common-uses-slag>.
Doney, Scott C. "The Dangers of Ocean Acidification." Scientific American 294.3 (2006): 58-65. Web.
Dudka, Stanislaw, and Domy C. Adriano. "Environmental Impacts of Metal Ore Mining and Processing: A Review." Journal of Environment Quality 26.3 (1997): 590. Web.
Electric Power Monthly. Rep. U.S Energy Information Administration, Oct. 2016. Web. 27 Sept. 2016. <http://www.eia.gov/electricity/monthly/pdf/epm.pdf>.
Emission Facts: Average Carbon Dioxide Emissions Resulting from Gasoline and Diesel Fuel. Rep. no. EPA420-F-05-001. U.S Environmental Protection Agency, Feb. 2005. Web. 27 Sept. 2016.
Federal Railroad Administration. National Rail Plan: Moving Forward. Rep. Federal Railroad Administration, Sept. 2010. Web. 27 Sept. 2016. <https://www.fra.dot.gov/eLib/Details/L02696>.
"From Ore to Steel." From Ore to Steel – ArcelorMittal. ArcelorMittal Steel, n.d. Web. 27 Nov. 2016. <http://corporate.arcelormittal.com/who-we-are/from-ore-to-steel>.
"Fuel Consumption by Containership Size and Speed." Fuel Consumption by Containership Size and Speed. Hofstra University, n.d. Web. 27 Nov. 2016. <https://people.hofstra.edu/geotrans/eng/ch8en/conc8en/fuel_consumption_containerships.html>.
Glaister, Bonnie J., and Gavin M. Mudd. "The Environmental Costs of Platinum–PGM Mining and Sustainability: Is the Glass Half-full or Half-empty?" Minerals Engineering 23.5 (2010): 438-50. Web. 24 Oct. 2014.
Grippo, Eric. Process for Manufacturing a Ceramic Element for a Watch Case and Element Obtained by This Process. Rolex SA, assignee. Patent US 7628894 B2. 18 Dec. 2009. Print.
Hewitt, Alistair. "The Ups and Downs of Gold Recycling." World Gold Council. World Gold Council, 05 Mar. 2015. Web. 27 Nov. 2016. <http://www.gold.org/supply-and-demand/ups-and-downs-gold-recycling>.
JohnsonMattheyPlc. "Johnson Matthey Platinum Group Metal (pgm) Refining." YouTube. YouTube, 30 Mar. 2012. Web. 27 Nov. 2016. <https://www.youtube.com/watch?v=E24bfF760sA>.
Khan, Rahil. "Different Types of Iron Ore - Mineral Processing & Extractive Metallurgy." Mineral Processing & Extractive Metallurgy. 911 Metallurgist, 5 Oct. 2016. Web. 27 Nov. 2016. <https://www.911metallurgist.com/blog/different-types-of-iron-ore>.
Khan, Rahil. "Gold Smelting & Refining Process - Mineral Processing & Extractive Metallurgy." Mineral Processing & Extractive Metallurgy. 911 Metallurgist, 28 Feb. 2016. Web. 27 Nov. 2016.
Kuck, Peter. "Iron Ore Statistical Compendium." USGS Minerals Infromation: Statistical Compendium - IRON ORE. United States Government, 11 Jan. 2013. Web. 27 Nov. 2016. <http://minerals.usgs.gov/minerals/pubs/commodity/iron_ore/stat/>.
Lacerda, L.d., and R.v. Marins. "Anthropogenic Mercury Emissions to the Atmosphere in Brazil: The Impact of Gold Mining." Journal of Geochemical Exploration 58.2-3 (1997): 223-29. Web. 24 Oct. 2016.
@madehow.com. "Stainless Steel." How Stainless Steel Is Made - Material, Manufacture, Used, Processing, Parts, Components, Structure, Steps. MadeHow, n.d. Web. 27 Nov. 2016. <http://www.madehow.com/Volume-1/Stainless-Steel.html>.
"Measuring Greenhouse Gas Emissions from Transportation." EPA. Environmental Protection Agency, n.d. Web. 24 Oct. 2016.
Michaud, David. "Gold Chlorination Process by Miller - Mineral Processing & Extractive Metallurgy." Mineral Processing & Extractive Metallurgy. 911 Metallurgist, 8 July 2015. Web. 27 Nov. 2016.
Pollak, Steve. "Fuel-efficient Transportation: An Overview." MNN - Mother Nature Network. Mother Nature Network, 27 Oct. 2010. Web. 27 Nov. 2016. <http://www.mnn.com/earth-matters/energy/stories/fuel-efficient-transportation-an-overview>.
"Pollution Prevention and Abatement Handbook." World Bank Group, July 1998. Web. 24 Oct. 2016.
Prasad, P. M., T. R. Mankhand, and A. J.K Prasad. "Molybdenum Extraction Process: An Overview." NML Technical Journal 39.2 (1997): 39-58. Web. 27 Nov. 2016. <http://eprints.nmlindia.org/1690/2/39-58.PDF>.
Ricketts, John A. "How a Blast Furnace Works." How a Blast Furnace Works. Steel Works, n.d. Web. 27 Nov. 2016. <http://www.steel.org/making-steel/how-its-made/processes/how-a-blast-furnace-works.aspx>.
RTI International. Coke Production. Rep. no. 021426.001.001. U.S Environmental Protection Agency, Office of Air and Quality Planning and Standards, May 2008. Web. 27 Nov. 2016. <https://www3.epa.gov/ttn/chief/ap42/ch12/bgdocs/b12s02_may08.pdf>.
Stubbles, John. "The Basic Oxygen Steelmaking (BOS) Process." The Basic Oxygen Steelmaking (BOS) Process. Steel Works, n.d. Web. 27 Nov. 2016. <http://www.steel.org/making-steel/how-its-made/processes/processes-info/the-basic-oxygen-steelmaking-process.aspx>.
Valia, Harfarshan S. "Coke Production For Blast Furnace Ironmaking." Coke Production For Blast Furnace Ironmaking. Ispat Inland Inc, n.d. Web. 27 Nov. 2016. <http://www.steel.org/making-steel/how-its-made/processes/processes-info/coke-production-for-blast-furnace-ironmaking.aspx>.
Van Oss, Hendrik G. "Slag-Iron and Steel." (2004): 69.1-9.6. U.S Geological Survey, 2004. Web. 27 Nov. 2016. <http://minerals.usgs.gov/minerals/pubs/commodity/iron_&_steel_slag/islagmyb04.pdf>.
Worrell, Ernst, Lynn Price, and Nathan Martin. "Energy Efficiency and Carbon Dioxide Emissions Reduction Opportunities in the US Iron and Steel Sector." Energy 26.5 (2001): 513-36. Web. 24 Oct. 2016.