Design Life-Cycle
assess.design.(don't)consume
Alana Rufer
Professor Cogdell
DES40A
December 3, 2019
Materials of the Scissor Life Cycle
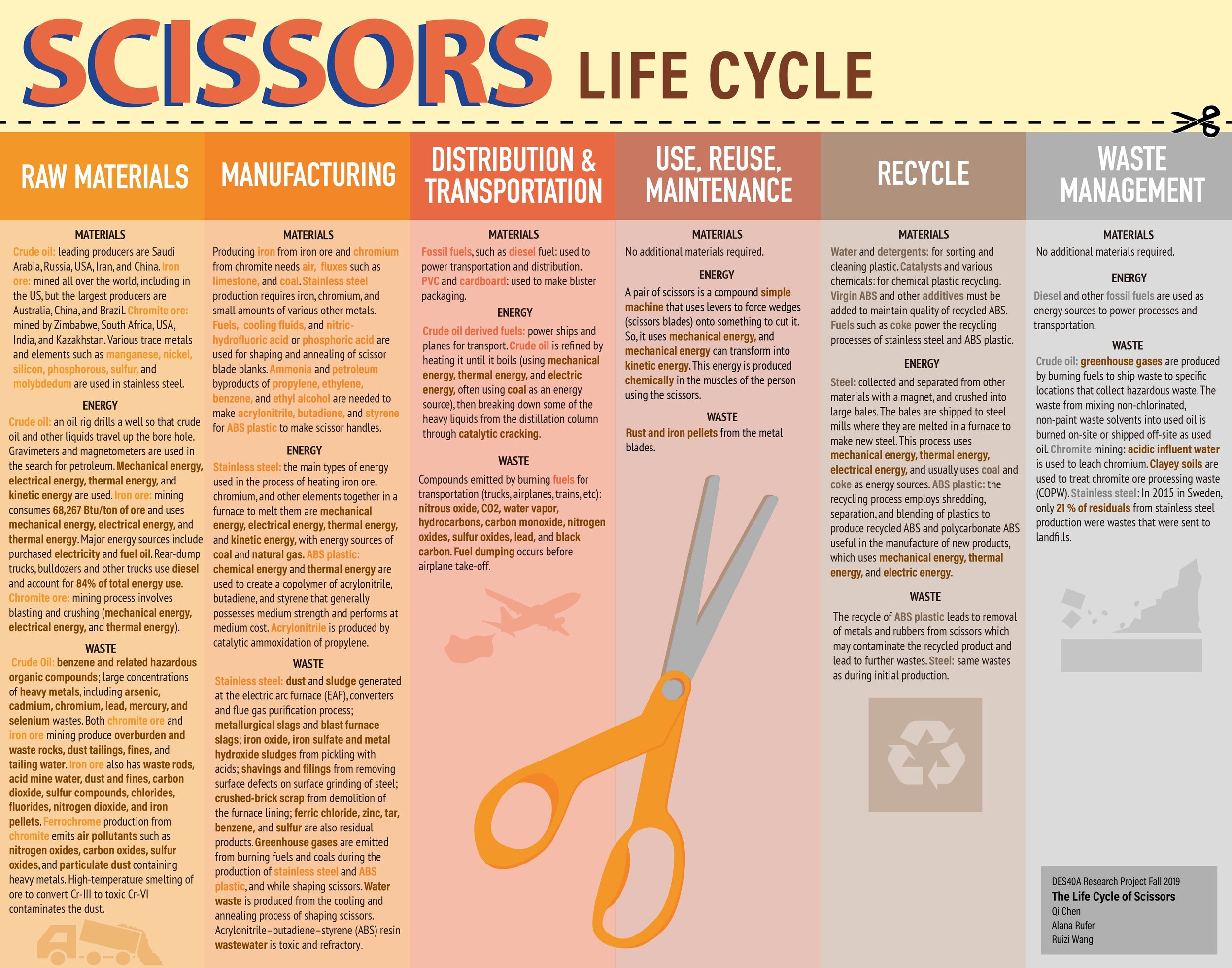
Scissors come in many varieties, from specialized tools for industry and crafts to household scissors. Common household scissors are produced by many different companies, including Fiskar, Acme, and Kaiser Group, though their basic forms and materials are very similar: metal blades with plastic handles, held together by a rivet to allow the blades to pivot and cut materials. Scissors consist principally of ABS plastic and stainless steel; however, the scissor life cycle––from extraction of raw materials, to manufacturing, packaging, distribution, and recycling or disposal––involves more materials than initially obvious, such as iron, chromium, crude oil, and fuels. Though materials alone do not provide a complete picture, they provide insight into the complexity of the scissor life cycle.
One of the first visible materials in scissors is ABS plastic, from which the handles are made. ABS is a copolymer composed of acrylonitrile, butadiene, and styrene, which are the source of the acronym ABS. It is categorized as a thermoplastic and has a molecular formula of C15H17N. In the manufacture of ABS plastic, monomers of acrylonitrile and styrene are polymerized in the presence of polybutadiene or a butadiene copolymer (“2-Propenenitrile, Polymer with 1,3-Butadiene and Ethenylbenzene”). Typically, styrene accounts for 50% of the material, though the amount of acrylonitrile and butadiene varies according to grades. Medium impact grade ABS, for example, consists of 57.4% styrene, 13.3% butadiene and 29.3% acrylonitrile (“2-Propenenitrile, Polymer with 1,3-Butadiene and Ethenylbenzene”). ABS is commercially manufactured in the US in large quantities. The amount of scissor materials triple just from those in the ABS, and, like most plastics, they originate in crude oil.
Acrylonitrile, butadiene, and styrene, while distinct components of ABS plastic, are all derived from petroleum. Acrylonitrile has the molecular formula C3H3N, and is mass produced in the US. Manufacturing acrylonitrile requires the reaction of propylene (one of many byproducts of petroleum refining), ammonia, air, and a catalyst under pressure at 400-510°C. This process converts 98% of the propylene, with a ratio of 1.1kg propylene consumed per 1kg acrylonitrile produced (“Acrylonitrile”). Butadiene (C4H6) is made either through dehydrogenation of butane or butenes, or through cracking materials from petroleum distillation (“Butadiene”). Styrene (C8H8) is industrially produced by dehydrogenation of ethylbenzene. Ethylbenzene is created by the reaction of ethyl and benzene, which are both derived from petroleum, which is derived from crude oil. Every year, over 15 million metric tons of styrene are produced (“Styrene”). These three processes all trace back to petroleum, making crude oil their main raw material.
Crude oil is a fossil fuel extracted and refined in many locations around the world, and the top producers include Saudi Arabia, Russia, USA, Iran, and China. To extract oil, an oil rig or platform must drill down into an oil reservoir, then pump the oil out. Afterwards, the oil is refined through distillation: crude oil is heated and condensed at different temperatures to separate components and impurities (“Petroleum”). ABS plastic, a main secondary raw material of scissors, relies primarily on this extraction of crude oil as a source of material.
The other major part of scissors––the blades and the rivet––consists of stainless steel. Steel making has two main approaches: BOF (Basic Oxygen Furnace) and EAF (Electric Arc Furnace). BOF accounts for 74% of the steel produced worldwide. In this process, iron and steel scrap are combined and heated to 1700°C by burning coke, which melts the metals and releases carbon to combine with the iron, creating steel. At the same time, oxygen is blown in to oxidize impurities, and other elements may be added to make alloys. Overall, BOF requires 600kg of coke (equivalent to 770kg of coal, from which coke is derived) to produce 1000kg of steel. EAF, on the other hand, uses mainly recycled steel, combined with some pig iron and direct reduced iron to ensure that the mixture is chemically balanced. The metals are heated to 1600°C by an electric current and impurities are removed from the melted metal (“How Is Steel Produced?”). Both processes create liquid steel which is cast and formed by hot rolling, then heat treated, and then annealed through heating and quenching to increase hardness. This creates a build-up on the metal called scale, which is removed either by pickling in nitric-hydrofluoric acid or by electrocleaning, which uses phosphoric acid and electricity. (“Stainless Steel”). After this step, forms can be cut out of the metal to make various products, such as scissors.
Stainless steel is an iron alloy characterized by the addition of chromium, which must compose 12-20% of the alloy for it to be considered stainless steel. Chromium contributes stain, rust, and corrosion resistance to steel, making it “stainless”. Combined with trace amounts of various other elements, including silicon, nickel, carbon, nitrogen, molybdenum, phosphorous, and manganese, the resulting metal is strong, durable, and sanitary (“Stainless Steel”). Different proportions of these trace elements alter the metal’s properties, creating over 50 standard grades of stainless steel. I could not ascertain the specific grade used in common household scissors, since there is no one specific alloy used in scissors; however, for comparison, hairdressing scissors generally use a 400 series steel, often 420 or 440C, which is hard due to their high carbon content (“Materials of Shears”). These steels are often manufactured in Japan and Germany, and consist respectively of 0.15% C, 12-14% Cr, and less than 1% Mn, Si, P, and S, or 0.95-1.2% C, 16-18% Cr, and less than 1% Mn, Si, P, S, and Mo (“Stainless Steel Grade Datasheets”). Since the composition of stainless steel varies and documenting the origin of all components is impractical for the purposes of this paper, only the origins of iron and chromium, the defining materials of stainless steel, will be detailed.
Iron, the main material used in stainless steel, is found and extracted in the form of ore. Iron ore is found in 5% of the Earth’s crust, spread almost everywhere on Earth (“ITP Mining”), and is mined in 50 countries, with Australia, Brazil, and China leading production (“How Is Steel Produced?”). In the US in 2000, 208 million tons of crude iron ore were produced. The preferred iron mining method is surface mining, though underground mining may also be used. Surface mining, also called “open-pit” mining, consists of removing soil, then drilling and blasting the ore underneath with explosives to break it down for ease of handling and transportation. Next, the ore is treated to remove impurities, called beneficiation, by being crushed, screened, ground, and concentrated using magnets, water, and chemical reagents to separate materials. The ore is then agglomerated into iron pellets (“ITP Mining”). In this process, coke, iron ore, and fluxes such as limestone are melted in a blast furnace by burning the coke, which, in addition to heating the ore, releases carbon monoxide that the ore reacts with. The resulting materials are drained and separated into slag and iron (“How Is Steel Produced?”). By the end of this process, the iron is sufficiently refined for use in stainless steel production.
The other key component of stainless steel is chromium, which is found naturally as chromite ore. Around the world, about 11 billion tons are mineable, with southern Africa estimated to contain 99% of the Earth’s chromite. In addition to South Africa, the US, Kazakhstan, and India are also some of the largest chromite mining countries in the world. Chromite deposits have varying characteristics, and as a result, different mining methods and amounts of beneficiation are necessary (“Chromium”). The processing of chromite uses coke as a source of carbon and heat to help extract chromium from Cr2O3. Then, when “carbon and Cr2O3 are combined in a molar ratio of 3:1 and subjected to increasing temperature, a number of oxidation-reduction reactions will ensue that will produce first a series of chromium carbides and finally, at 2,080°C (3,775°F), pure chromium and carbon monoxide” (Downing, et al). This often still contains impurities, which sometimes needs further refinement. Stainless steel production consumes the majority of chromium produced (“Chromium”), and this addition of chromium in steel defines stainless steel.
The general process of manufacturing scissors from their secondary materials has two main steps: creating the plastic handles and creating the stainless steel blades, which are assembled together and secured by a rivet. Metal scissor halves, called “blanks,” are cut from sheets of stainless steel through cold stamping or drop forging (“Stainless Steel”). The blanks are trimmed and drilled through to create a hole, then hardened and tempered through heat treatments, and any warping is straightened out. Next, to create the blades of the scissors, the blanks are grinded and polished. During this step, they are cooled with water and other fluids to prevent additional warping from heat (“Scissors”). The scissors handles are made by injection molding plastic. This begins with heating and melting a thermoplastic, such as ABS in the case of scissors, then injecting it under pressure into a mold, maintaining pressure until the plastic cools and solidifies, and finally removing it from the mold (Rosato 2). Once the blades and handles are finished, the blanks are inserted into slots in the handles with adhesives hold the components together, though I could not ascertain the type of adhesive used. Next, a rivet or screw is inserted through the holes of the blanks to fasten the two halves of the scissors together and serve as a hinge for the scissors to open and shut. Lastly, the scissors are adjusted to ensure the blades align properly for effective shearing (“Scissors”). Though this concludes the manufacture of the scissors themselves, there are still many more materials involved in the following parts of the life cycle.
After their manufacture, scissors are packaged, often with blister packaging. This uses additional materials such as PVC plastic and cardboard. The plastic is shaped through thermoforming, in which a sheet of thermoplastic is heated and pushed into a mold using a vacuum (“Thermoforming Process”). The molded plastic is die cut and attached to a backing made of paperboard or plastic, forming a pocket that holds the scissors (Dube). I could not find the exact type of thermoplastic used in scissor packaging, especially since different brands use different packaging, but PVC is commonly used. Overall, the packaging is recyclable, since its components of cardboard and PVC are recyclable. PVC can be recycled mechanically, in which the plastic is sorted, ground, cleaned with detergent and water, melted, and cast. Or, it can be recycled chemically, in which the polymer is broken down with heat, catalysts, and other chemicals. However, this process downcycles the materials due to the deterioration of the properties of the plastic (Sadat-Shojai, et al). Even so, since the consumer is responsible for disposing of these materials, they may not always be recycled. European countries and the US have low plastic recycling rates, and usually incinerate waste and use landfills, where the chlorine content of PVC creates a hazard (Sadat-Shojai, et al). These materials used for packaging, though not directly a part of scissors, contribute to the life cycle’s complexity.
Once manufactured and packaged, the scissors are shipped to stores. This is part of the extensive transportation involved in the life cycle, which consumes a significant amount of fuels. For example, chromite, which is mostly found in southern Africa (“Chromium”), and iron ore, which is found all over the world, must be transported from mines to a common location to make stainless steel. In US iron mines, diesel fuel powers local ore transport, and transport from the mine is done by rail and ship (“ITP Mining”). Transporting materials to factories, such as Kai Group’s factory in Japan (“Factories’ Profiles”), and then to stores consumes yet more fuel. It is difficult to find exact modes of transportation and fuels used, but these widespread locations suggest that transport and manufacturing processes require substantial fuel consumption.
No new materials are used during the use of scissors since households typically do not repair or otherwise maintain them, and at the end of their life they either recycled or otherwise disposed of. For recycling, ABS plastic and stainless steel must be processed separately. ABS recycling begins by shredding used plastic, then separating plastics and metals either with near infrared technology or with streams of water at different speeds. It is then ground, purified, and melted into a secondary material (“Recycling of Plastics”). A drawback to this process, however, is that the mechanical and thermomechanical properties of the resulting plastic degrade, even when virgin ABS and other additives are added to counteract these effects (Scaffaro, et al). Stainless steel, on the other hand, is theoretically completely recyclable, and indeed 60% of stainless steel produced in 2007 was made from recycled material (“Environmental Aspects of Stainless Steel”). The basis of the primary methods of stainless steel production, BOF and EAF, support this circular cycle, since they respectively use up to 30% or 90-100% recycled steel as inputs (“How Is Steel Produced?”). However, despite their recyclability, scissors may still be thrown away in the trash, reaching their end in a landfill due to negligence or ignorance of the consumer. So though in theory, the constituent materials of scissors are recyclable, the implementation and quality of the processes have room for improvement.
Thus, throughout the life cycle of common household scissors, the materials involved in go far beyond those visible at face value. The plastic and metal of scissors trace back to raw materials of crude oil, ores, coal, and a multitude of other materials used in manufacturing and transportation, and as the life cycle progresses, this list becomes increasingly complex and interconnected. This illuminates a high level of worldwide interdependence and material use inherent in modern manufacturing. A large amount of these materials are finite resources. And, though many are, to an extent, recyclable, many are sent to landfills, terminating their journey in a grave instead of a cradle. Therefore, they contribute to an unsustainable manufacturing process. Despite the ostensible simplicity of scissors, the materials used behind the scenes reveal a complex life cycle and environmental impact.
Bibliography
“Acrylonitrile.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, https://pubchem.ncbi.nlm.nih.gov/compound/Acrylonitrile.
“Chromium.” Minerals Education Coalition, Minerals Education Coalition, mineralseducationcoalition.org/minerals-database/chromium/.
Downing, James H, and Frederick E Bacon. “Chromium Processing.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 23 Aug. 2013, www.britannica.com/technology/chromium-processing.
Dube, Nathan. “Carded Packaging: The Difference Between Blister, Skin And Clamshell Packaging.” Industrial Packaging, Industrial Packaging, 2 May 2019, www.industrialpackaging.com/blog/carded-packaging-difference-between-blister-skin-and-clam-shell.
“Environment Aspects of Stainless Steel.” British Stainless Steel Association, BSSA, www.bssa.org.uk/sectors.php?id=99.
“Factories' Profiles.” KAI Factory, KAI Corporation, www.kai-group.com/global/en/kai-factory/profile/.
“How Is Steel Produced?” World Coal Association, World Coal Association, 22 Feb. 2019, www.worldcoal.org/coal/uses-coal/how-steel-produced.
“Materials of Shears.” Bold Barber, Boldbarber.com, 15 Jan. 2016, boldbarber.com/materials-of-shears/.
“Petroleum.” National Geographic, National Geographic Society, 14 Jan. 2013, www.nationalgeographic.org/encyclopedia/petroleum/.
“Recondition and Recycle Used Scissors and Shears.” Eberly Scissors and Shears Reconditioning Service, John A Eberly, Inc, jaeberly.com/id3.html.
“Recycling of Plastics.” Heathland, Heathland, https://www.heathland.com/?page_id=7&lang=en.
Rosato, D V. Injection Molding Handbook. Springer US, (2012).
Sadat-Shojai, Mehdi, and Gholam-Reza Bakhshandeh. "Recycling of PVC wastes." Polymer degradation and stability 96.4 (2011): 404-415.
Scaffaro, R., L. Botta, and G. Di Benedetto. "Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles." European Polymer Journal 48.3 (2012): 637-648.
“Scissors.” How Products Are Made, Advameg, 2019, www.madehow.com/Volume-3/Scissors.html.
“Stainless Steel.” How Products Are Made, Advameg, www.madehow.com/Volume-1/Stainless-Steel.html.
“Stainless Steel Grade Datasheets.” WorldStainless.org. Atlas Steels Technical Department, Aug. 2013, http://www.worldstainless.org/Files/issf/non-image-files/PDF/Atlas_Grade_datasheet_-_all_datasheets_rev_Aug_2013.pdf
The Editors of Encyclopaedia Britannica. “Butadiene.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 09 Nov. 2011, https://www.britannica.com/science/butadiene.
The Editors of Encyclopaedia Britannica. “Styrene.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 19 Feb. 2019, https://www.britannica.com/science/styrene.
“Thermoforming Process.” VisiPak, VisiPak, https://www.visipak.com/thermoforming-process.html.
“2-Propenenitrile, Polymer with 1,3-Butadiene and Ethenylbenzene.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine, https://pubchem.ncbi.nlm.nih.gov/compound/acrylonitrile-butadiene-styrene.
United States Department of Energy, Office of Energy Efficiency & Renewable Energy. “ITP Mining: Energy and Environmental Profile of the U.S. Mining Industry: Chapter 4: Iron.” Energy.gov, BCS, Inc, 2002, pp. 1–13, https://www.energy.gov/sites/prod/files/2013/11/f4/iron.pdf.
Ruizi Wang
DES 40A
Professor Cogdell
2 December 2019
The Life Cycle of Scissors: Embodied Energy
Convenient tools have always been an integral part of our lives, and seemingly small tools can often help us solve severe problems, sometimes they can make our lives or work easier. For this life Cycle project, our team chose scissors as the object of our research because scissors are very close to our lives, and almost everyone's home has such a convenient tool. And people use scissors to cut different kinds of things every day. Although scissors seems like the small tool, from finding raw materials, design, production to people using it to recycling and waste, this seemingly small scissors also consumes enormous energy in the production process. However, for the following contents, I will explain all the information I found regarding the embodied energy in the life cycle of scissors.
Before start making scissors, getting raw materials can consume a lot of energy. The raw materials can include Iron ore, chromite ore, plastic, and other metals and elements like nickel, silicon, phosphorus, molybdenum. During the ore mining process, continuous blasting, crushing, consigning and drilling are required. The major energy sources include purchased electric energy, mechanical energy and fuel oil. Because in the ore mining process(iron and chromite), it requires to use many machines such as rear-dump trucks, bulldozers service, and bulk trucks account for 84 percent of the total energy. The Energy and Environmental Profile of the U.S. Mining Industry states that iron ore mining consumed 62.3 trillion Btu in 1992. Also, the fuel oil consumed 669.6 thousand bbl and the electricity purchased 7300 million kWh in 1992("Iron"). After ore mining, the extraction is essential and consumes much energy.Stainless steel is used to make scissors, which have plastic handles on metal blades. “Stainless steel is made of iron, about 1% carbon and at least 10% chromium.” It has the advantages of lightness and rust prevention. The handles of stainless steel scissors made of strong, lightweight materials such as ABS (acrylonitrile-butadiene-styrene) plastic(“Scissors”). The steel manufacturing process begins with the smelting of iron ore (Fe2O3)in a blast furnace. This smelting process melts the iron and separates it from the original rock material. Iron ore is mixed with coke, which is a blast furnace that burns coke to heat the iron ore and react it to iron (Fe2), nitrogen (N2) and carbon dioxide (CO2), and produce "pig iron" (Martelaro 2016). Mechanical energy, thermal energy and chemical energy are used in this process. From this process, the "pig iron" can used for creating steel. For making steel, it should use machines and high temperatures(1800+°C) to release the carbon from the iron. It can use basic Oxygen Furnace to do that. The energy is about 1.5 million Btu per ton of steel is required in steelmaking. The remaining energy is provided by the oxidation reaction produced by the oxygen lance(Luther 2016). Oxygen can increase the temperature and have a reaction with carbon and then reduces the carbon of iron to produce carbon steel. So this process consumes mechanical energy, also converting thermal energy into chemical energy. Stainless steel still need chromium, after the carbon and Chromium(III) oxide combined at a molar ratio of 3:1 and use high temperatures to heat them, the reduction reaction will happen and producing pure chromium and carbon monoxide at 2080°C(Downing & Bacon 2013). This process also converts thermal energy into chemical energy. At final steps, use an electric arc furnace to melt solid pig iron and scrap steel. According to analysis, the energy consumption of electric arc furnace are from 350-700kWh/ton of steel produced(Luther 2016). After heat the metals, the liquid steel has been created. The liquid steel can use to make the products. This process includes mechanical energy and convert thermal energy into chemical energy. "The production of pig iron is the most energy-intensive process for the production of steel." It counts 13.5 × 109 joules per ton (1000 Kg) of pig iron produced and Electric arc furnace reduces energy consumption at 2.25 × 109 Joules per ton(Martelaro 2016). For Acrylonitrile Butadiene Styrene(ABS) plastic handles of the scissors, is an "opaque thermoplastic and amorphous polymer" (Rogers 2015). Plasticizing ABS plastic requires high-temperature heating to cure it. This process uses thermal energy.
After these raw materials are ready, the process of making scissors can begin. Through the Kai factory website, it shows that the steps of the production process. The first step is laser cutting. They need to control computer program to cut out a blade from the stainless steel material with a laser beam. The computer uses mechanical energy and electric energy, through the energy calculator website, it states that "the average for modern desktop P.C. can use approximately 100 watts of power for a day". And in the process of use laser beam, it converting electric energy into mechanical energy, then converting mechanical energy into thermal energy. According to the data on the Epilog laser website, it writes that "the typical maximum power consumption of the laser is 1900 watts". It states that "the laser costs, roughly, just under 15 cents per hour to run the laser or $1.20 per eight hour day." However, it will cause in energy-efficient operation and cost-effective(epiloglaser 2019). After the laser cut, the blade should be put into the electric furnace with 1000ºC heat, them make it cold in the water, in the end annealing the blade at 180ºC. Through the analyses, the electric furnace consumes 18,000 Watts for 2 hours a day, and "energy consumption for a continuous strip annealing line ranges from 1-1.5 MMBtu/ton" (Luther 2016). The thermal process converted mechanical energy and electrical energy into thermal energy. Additionally, The blade needs grinding, polishing, blade edging and shot blasting to make it smoother and sharper. It should grind the blank into a blade by applying the edge to a fast-moving abrasive belt or wheel. I cannot find the exact numbers for energy consumption of grinding and polishing machine, but both grinding and polishing convert electrical energy into mechanical energy and then into kinetic energy. The next step should be making the handles, the material for making that is ABS plastic The plastic are made by injection molding. During this process, the molten plastic is pressed into a handle-shaped mold under pressure. Due to pressure, potential energy is converted into kinetic energy. The mold releases thermal energy and then cools and solidifies the ABS plastic. During this time, thermal energy is released. Once the plastic is fully cured, it can open the mold and remove the handle. The handle has a hollow slot into which the tip of the blade can be inserted. And use strong adhesive to attach the handle. Through the research, more finding is that structural adhesives of acrylic and methyl methacrylate have high tensile strength and good adhesion to metal and plastic(2018).To attach the handle and the steel, the cured adhesive must be heated above 200 ° C to soften it and the parts should be pried open while the adhesive is still hot. It's a conversion of mechanical energy into thermal energy. Moreover, researchers in 3M Industrial Tapes and Specialties gives information states that "Mechanisms for adhesion include both mechanical adhesion and specific adhesion. Mechanical adhesion occurs when the adhesive flows into the texture of the substrate." (1997) And the tackiness of acrylic pressure-sensitive adhesives is due to the longer time it takes for the adhesive to flow into and into the surface texture, and the adhesive polymer aligns with the surface to form an interaction. The two polished blades had connected through rivets or screws through previously drilled holes. This process uses automatic screwing machine; it can save energy because after the combination of parts punching and screwing, the automatic screwing machine is entirely driven by mechanical transmission, which saves the energy of the special screwing machine and mechanical energy for tapping screws. Furthermore, the machine can save labor cost through in-mold automatic screwing machine combines screwing and punching of parts into one process for full automation.
After making the scissors, the goods need to transport to various countries or cities for sale. Transportation in modern society is developing very fast, and cargo can be transported by truck, plane, train, and ship. Different ways of transportation also determine the energy consumed during transportation. During the transportation, it consumes a lot of fuel, such as diesel and gasoline. For making fuel, the process includes refining crude oil is to heat it until it boils, it uses mechanical energy to convert thermal energy. Additionally, through catalytic cracking,breaks down some of the heavy liquids from the distillation column(chemical reaction). According to the statistics from eia.gov, the energy consumption estimation of transportation in the USA is 18973 trillion Btu in 2019. What's more, the U.S. uses 28% of its total energy annually to move people and products from one place to another.
By shipping scissors, people then started buying and using scissors to do works. A pair of scissors is a simple compound machine that uses levers to force wedges (scissors blades) onto something to cut it. So it uses mechanical energy, and mechanical energy can transform into kinetic energy.
Most of the materials used to make scissors are recyclable, which can save more energy use, and it's great for environmental protection. Bureau of international recycling explains that the demand for stainless steel has doubled, and production has increased to more than 25 million tons per year in the past ten years(BIR). In this case, the recycling industry has become an important player in providing stable high-quality secondary raw materials. After For the process of recycling stainless steel, the first step is to separate the steel from other materials by a magnet. Secondly, compact stainless steel products to pieces, because it can make the transportation and handling more convenient and this process converting the mechanical energy to kinetic energy. After that, they are cutting stainless steel into small pieces with a hydraulic machinery. This process converts electrical energy to mechanical energy, and the mechanical energy converted to hydraulic energy. They operate at pressure in 100s of bars. It's like 100 times the normal atmospheric pressure. The process also requires use shredders to separate ferrous metal from other materials. Most shredders include at least two tine gears that rotate relative to each other. At the final step, melt the recovered materials together in the furnace, then the molten stainless steel is poured into a casting machine and formed into steel ingots. After that, they can be rolled into flat plates for manufacturing new products. It consumes electric energy, and convert mechanical energy to thermal energy. "Researchers estimate that recycling saved 33 percent of the energy needed to produce 17 million tons of stainless steel globally in 2004; with 100 percent recycling, however, stainless steel production would use 67 percent less energy." (Brady 2016) These data demonstrate the importance of recycling the stainless steel. The other main component of the scissors, ABS plastic, is also 100% recyclable. This process uses shredding, separation and blending of mixed plastics to produce recycled ABS (acrylonitrile butadiene styrene) and PCABS(polycarbonate ABS) that can be used to make new products, and it uses a series of steps to remove unwanted components. However, the process converts electrical energy to mechanical energy. Since a lot of materials can be 100% recycled, the waste management for scissors will not consume much energy. Both waste steel and ABS plastic had processed through mechanical energy, electrical energy, and thermal energy during the recycling process.
In conclusion, through all of my research, we learned that a small household convenient tool consumes a lot of abundant energy in the life cycle, also shows that the production cost of scissors is not low. Among them, making steel used a large amount of energy, it can conclude that the steel in our life is efficient and needed. On the other hand, although production consumes much energy, but the materials that were used in the production of scissors can be fully recycled, which saves a lot of cost and energy and great for environmental protection.
Bibliography
“Iron.”Energy and Environmental Profile of the U.S. Mining Industry, 14 November, 2019,https://www.energy.gov/sites/prod/files/2013/11/f4/iron.pdf
“Scissors.” How Products Are Made, Advameg, 2019, www.madehow.com/Volume-3/Scissors.html.
Martelaro, Nikolas. “Energy Use in US Steel Manufacturing.” Energy Use in US Steel Manufacturing, 4 Dec. 2016, http://large.stanford.edu/courses/2016/ph240/martelaro1/.
Luther, Robb. “Basic Oxygen Furnace Energy...” HeatTreatConsortium.com, HeatTreatConsortium.com, 30 Jan. 2016, http://heattreatconsortium.com/metals-advisor/basic-oxygen-furnace/basic-oxygen-furnace-energy-consumption/.
Downing, James H., and Frederick E. Bacon. “Chromium Processing.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 23 Aug. 2013, https://www.britannica.com/technology/chromium-processing.
Rogers, Tony. “Everything You Need to Know About ABS Plastic.” Everything You Need to Know About ABS Plastic, 13 July 2015, https://www.creativemechanisms.com/blog/everything-you-need-to-know-about-abs-plastic.
“Manufacturing Processes (SCISSORS): KAI FACTORY: KAI Group.” KAI FACTORY ,KAI Group, https://www.kai-group.com/global/en/kai-factory/process/scissors/.
“Electricity Usage of a Desktop Computer.” Electricity Usage of a Computer - Energy Use Calculator, http://energyusecalculator.com/electricity_computer.htm.
“Power Consumption and Your Laser.” Power Consumption and Your Laser, 29 May 2019, http://support.epiloglaser.com/article/8205/29954/power-consumption-and-your-laser.
“Strong Adhesives for Bonding Metal, Glass & Plastic.” Permabond, 1 Aug. 2018, https://www.permabond.com/2016/06/01/strongest-adhesive/.
“U.S. Energy Information Administration - EIA - Independent Statistics and Analysis.” U.S. Energy Information Administration (EIA) - Consumption & Efficiency, 2019, https://www.eia.gov/consumption/.
“What Exactly Is the Physical or Chemical Process That Makes Adhesive Tape Sticky?” Scientific American, Scientific American, 14 July 1997, https://www.scientificamerican.com/article/what-exactly-is-the-physi/.
“- BIR - Bureau of International Recycling.” BIR, https://bir.org/industry/stainless-steel/.
Brady, Angela. “How to Recycle Stainless Steel and Aluminum.” Home Guides | SF Gate, 7 Oct. 2016, https://homeguides.sfgate.com/recycle-stainless-steel-aluminum-79108.html.
“POLYMER WASTE MANAGEMENT.” Search Results for AbS, https://polymerwastemanagement.blogspot.com/search?q=AbS.
Qi Chen
DES40A
Professor Cogdell
4 December 2019
Environmental Approaches are Involving in Scissor’s Life Cycle
Scissors are products that are commonly used these days. There are diverse scissors coming out due to the applications of human-centered designs. The production of scissors benefits people to organize their works. While the byproducts and wastes have potential impacts on the environment and animals, many companies are making efforts to utilize the byproducts or come up with innovations of chemical processes to confront the appeals of environmental friendly. Some transportation companies also conduct experiments for “green” fuels.
Scissors are mainly made of stainless steel and acrylonitrile butadiene styrene(ABS) plastic. To make stainless steel, manufacture companies import raw materials from mining companies that mostly from South America and Asia. Unlike steels that are products of high concentrations of carbons and iron(Goyal, “What Is the Difference Between Steel and Stainless Steel?”), the stainless steels have low concentrations of carbon and are alloys that mainly composed of iron ore, chromite ore and nickels, which helps scissors to resist oxidization for long-time use, and that go through machines that convert mechanical energy and electrical energy into heat to blend metals together under high temperatures. After shaping stainless steel into sheets, manufacture companies export them to scissors companies that shear sheets with specific moulds into blades. The other component of scissors, ABS plastic, is “a terpolymer formed by polymerization process styrene and acrylonitrile in the presence of polybutadiene( “ABS Plastic Production, Price and Market Demand.”)” and is highly recyclable. It is also easier and cheaper to manipulate than stainless steel does; they require less energy and low-cost raw materials to heat up, react and shape. When both components are ready, workers in scissors companies assemble them together and pack them up with PVC and cardboards. Finally, they are shipped globally. After long-time operations of scissors, some of them are oxidized or broken, and they are returned to companies to recycle or transported to landfills as wastes.
Wastes From Production of Stainless Steel
Even though the overall life cycle of scissors is simple, there are many wastes and byproducts during every process. Due to the need of chromium from chromite ore, the production of stainless steel induces more wastes for raw materials. The general process of extraction and beneficiation of iron and chromite ores are similar( as shown in Figure 4-1), so many of their wastes are quite the same.
As the mining process of iron ores, mining chromite ores creates waste rocks, dust, tailings and tailing waters (MiningWatch Canada ii). The waste rocks contain overburdens, which is “the material other than the mineral generated during the process of excavation ( Nilima et al. 400)” and mostly end up in the landfill as wastes,
(Department of Environment 4-7)
and development rocks, which is “byproduct of mineral extraction in underground mines (400)” and will be used for further extractions of less-pure metals. Overburdens themselves are not toxic, however, “when these are disposed on useful and arable lands for indefinite period, it leads to its degradation. Apart from this, they also degrade adjoining lands sometimes due to leachate, change of local drainage pattern and groundwater conditions. (400-401)” Tailings are mostly heavy metals, and their contents are similar for ores. For iron ores, tailings contain heavy metal ions such as copper, lead, zinc, chromium, tin, molybdenum and uranium(402), which are also tailings of chromite ores. Once heavy metals contaminate the wastes, such as tailing waters and dust, they will contaminate the ecosystem, like greenlands and rivers, rapidly that organisms can no longer live in those areas. As reports by Buzz, “Mining ore can also lead to acid rock drainage. Acid gets created when water and oxygen interact with sulphur-bearing minerals and chemicals within the rocks,(‘Ore Conservation is a Major Benefit When Scrap Metal Gets Recycled’)” which will also result in undesirable environments for animals,especially for fish, to live in.
Despite the tailings from mining, the residues from beneficiations of both ores are also hazards to ecosystems. During beneficiation, for iron ores, the impurities, “the undesirable chemical components, such as phosphorous, sulfur, sodium, potassium (alkalis), alumina, silica and sometimes titanium (Department of Energy 4-4)” are removed and then they are concentrated into different products due to different density( as shown in the Figure 4-1). Most of the chemical components, like sulfur and metal ions, will lead to acidity of lands and can only be recovered after long-period of time. While for chromite ores, after removal of impurities, they have to undergo chemical reactions to form alloys with other metals and then they are concentrated to form different products. Ferrochrome is the most common alloy that combines iron and chromium and is used to form stainless steel. Due to extra steps taken to beneficiate chromite ores, the residues vary greatly from iron ores. The ferrochrome production “emits air pollutants such as nitrogen oxides, carbon oxides and sulfur oxides (NOx, COx, SOx) and particulate dusts that contain heavy metals such as chromium, zinc, lead, nickel and cadmium (MiningWatch Canada ii).” Additionally, according to the reports from Mining Watch Canada, “during the high temperature smelting of chromite ore, some Cr-III is converted to toxic Cr-VI” and “ some processes, such as milling and agglomeration (i.e. sintering) may also produce Cr-VI (ii)”, which can contaminate most of the wastes and byproducts, including slags, dusts, tailing waters and air pollutants, and eventually reach the farmlands that threat human health and habitats of all organisms on those areas. Sometimes sulphuric acids can also be result from beneficiation. Buzz shows in his or her article that “since the beneficiation process requires dissolving the minerals surrounding the ore” and the process “releases chemicals in the rock. Those chemicals can go into nearby streams, freshwater bodies, and into the atmosphere.”
The wastes of production of stainless steel are not merely from raw materials. The further manufactures of stainless steels also cause wastes. “During the stainless steel production process, wastes are generated at the electric arc furnace (EAF) and at the converters. These wastes are a problem for stainless steel industries, for they must be disposed of in controlled landfills. Finding areas to make more landfills is becoming difficult, and new landfills are being placed far from the industries. This leads to an increase in disposal prices and, furthermore, in production prices. (P. J. Nolasco-Sobrinho, et al.)”
Waste Management, Recycle and Environmental Approaches of Stainless Steel
Facing both environmental and economical problems for productions of stainless steels, industries are coming up with solutions that try to manage their wastes, minimize the pollutions and utilize their wastes for secondary use or recycle. Reports from Nilima shows that iron ore has a high and fluctuate stripping ratio between 1-3: 1, which means for every ton of iron ore produced, one to three times of waste rocks are generated. According to studies done by the Nilima and his partners, some mining companies found that their waste rock, which “is disposed of in piles located near the mine(400),” “also can be used in dams or other on- or off-site construction(400).” Once those mining companies operate, manage, examine and determine the potentials for waste rocks from raw materials like iron ores, they, especially for India where “iron ore mines are the biggest contributor to total mining wastes(Nilima el at. 400)” and is one of the biggest exporters of ores, are able to reduce their cost for landfills and reduce pollutions to the local ecosystems. Furthermore, some companies and people like Unlü K and Haskök S, develop experiments to maximize their removals of Cr(IV) that are irreversible chemicals during the processing of chromite ore. Unlü K and Haskök S in their article “Treatability of chromite ore processing waste by leaching.” describe their experiments, data analysis and the results that the acidic influent water is the most effective to remove the Cr(IV), which potentially benefits mining companies to improve and develop more useful and efficient strategies to prevent toxic Cr(IV) to get into ecosystems.
Moreover, for the companies that produce stainless steel, the studies by Eva found out that “the iron oxide that is obtained on recycling of hydrochloric acid may, under certain conditions, be sold to the electronics industry as ferrite,” and the “iron sulphate, which is a by-product on the regeneration of sulphuric acid (pickling liquor) is used for water, increasing the efficiency of purification(‘Steel Production Residues’).” The ballast and steel slag asphalt that are produced during recycling and production of stainless steel can also be utilized in areas like road construction and building industry(Eva). In Eva’s report, the applications of residues from recycling steels are far more than these; as she stated in the report “no less than 80 per cent of the residual products are utilized,” the scrap metal can be reused within companies as iron raw material; “at the iron-ore based plants,” “a large portion of the residual products can be fed back into the processes”; “The briquettes” from reburning and shaping steels can “render a raw material for the blast furnace that includes iron, limestone and carbon.” Eventually, only 21% of the residues goes to the landfill after recycling( Eva).
Waste from Production of ABS Plastic
Besides stainless steel, acrylonitrile–butadiene–styrene (ABS) plastic is the other essential material for scissors. ABS plastic is used in handles and junctions depending on different usages. As Hosch states in his article “Acrylonitrile-Butadiene-Styrene Copolymer: CHEMICAL COMPOUND,” ABS plastic is a “hard, tough, heat-resistant engineering plastic that is widely used,” and it is “essentially a styrene-acrylonitrile copolymer modified by butadiene rubber, ABS combines the resilience of polybutadiene with the hardness and rigidity of polyacrylonitrile and polystyrene.” It is also inexpensive and “has a strong resistance to corrosive chemicals and/or physical impacts(Tony),” which allows it to be highly demanded by many companies. Despite all the advantages of ABS plastic, the wastes and byproducts from production of the plastic are remained as problems. One of the biggest problems is the wastewater from manufactures. The wastewater is a complex, toxic and refractory industrial water(Yuexi, Zhou et al.) and once it reaches the streams and rivers, local ecosystems will be destroyed.
Waste Management, Recycle and Environmental Approaches of ABS Plastic
Although the wastewater from manufacturing ABS plastic is a severe problem, there are useful experiments conducted to uncover the mechanisms. Studies done by Yuexi and her partners has successfully discovered the key pollutants, mononuclear aromatics and acrylonitrile dimers, in the wastewater by comprehensive analysis of gas chromatography spectrometry with a mass or flame ionization detector. Besides, the same team also develop useful method to treat the wastewater. In their article “Treatment of wastewater from acrylonitrile–butadiene–styrene (ABS) resin manufacturing by Fe0/GAC–ABFB”, they figured out that when Fe0/GAC reactor was performed by ABFB reactor, the removal efficiency can be very high, which is up to 90-95%, which will allow ABS plastic production companies to minimize their environmental impacts if this method is widely used.
Despite the fact that ABS plastic is highly recyclable, it may be contaminated by extrusion when it is removed from products that contain metals, including scissors. Students in Anna University, fortunately, developed methods to avoid and remove the contamination. The steps include: 1.Shredded ABS blend is separated from metallic impurities by magnetic separator; 2.The flakes are then washed in a specific gravity tank to separate the blend based on their density; 3.Separated flakes are washed in a wash tank and air-dried; 4.ABS recovered from the process is re-processed in an extruder and re-used for secondary engineering applications.
Waste from Transportations
Raw material productions and manufactures are large portions of scissors’ life cycle, while transportation is also important. During minings and beneficiations, transportations like trunks and trains are required to ship raw materials to specific machines or locations. After concentration and transformation of the raw materials, materials are transported to scissors’ companies for secondary manufactures. After reshaping the stainless steel and ABS plastic, transportations are required to ship blades or complete piece to further assembling and packaging. When all scissors are completely packed, transportations are required to ship scissors all over the world. Last but not the least, when scissors come to recycle, they also need to be sent to the companies individually or together by certain transportation.
Transportations provide convenience in a wide area, but they also lead to air and water pollutions and irreversible damages to the environment due to their use of fuels. The fuels used by transportations are mostly originated from crude oil. Crude oil, according to the report from Minnesota Pollution Control Agency(MPCA), “contain enough benzene and related organic compounds to make them a characteristic hazardous waste(1),” and “ they may also contain hazardous concentrations of heavy metals, including arsenic, cadmium, chromium, lead, mercury, and selenium(1),” which can be fatal to both animals and plants. When vehicles burn the fuels that made from crude oil , they also generate greenhouse gases like carbon dioxide. Daniel has recorded in his article “How Gasoline Becomes CO2” that for car to burn one gallon will release about 19 pounds of carbon dioxide; in the article “How Much Fuel Does an International Plane Use for a Trip?” the author states that for “a plane like a Boeing 747 uses approximately 1 gallon of fuel (about 4 liters) every second” and “over the course of a 10-hour flight, it might burn 36,000 gallons (150,000 liters)”, which is similar to international flight from China, where most of the raw materials of scissors come from, to the United States, where many manufactures of scissors take place; the total emissions of carbon dioxide, therefore, is 684,000 pounds, which is a very huge amount. While there are still a large number of transportations in use at the same time, the greenhouse gasses emissions and the damages to the environment are hard to estimate. Daniel also points out something that is even worse, “sometimes there’s not enough oxygen available to complete the reaction, in which case hydrocarbons can be converted into poisonous carbon monoxide (CO),” which can cause heart and lung disease when it accumulate to a certain amount.
Waste Management, Recycle and Environmental Approaches of Transportations
Due to the hazardous characteristics, the wastes from crude oil are required to transported to certain organizations like MPCA, Licensed Paint Collection Site (LPCS), Very Small Quantity Generator Collection Program (VSQGCP) or Household Hazardous Waste Collection Program (HHWCP) depending on the quantity of the wastes. There are solutions coming out to treat the wastes that some of the wastes are recycled by mixing non-chlorinated, non-paint waste solvents into used oil burned on-site or shipped off-site as used oil(MPCA), which reduces pollution to the environment.
With the advanced in technology, engineers also try improve vehicle themselves to reduce pollution and wastes. Parker and his partners, for example, develop aero-engines that help reduce the emission of greenhouse gases. Honeywell UOP develops green jet fuel that “reduce greenhouse gas emissions by 65-85% compared with petroleum-based fuels, based on UOP’s lifecycle analysis.” Recycling airplane pieces from aircraft or airplane that no longer use can also help to protect the environment and economy, just as Rick mentions in his article that engines are recyclable and can cut cost if they are recycled.
In conclusion, scissors are composed of two main materials, ABS plastic and stainless steels, and their general life cycle is simple. Removing rust and iron pellets from stainless steels and contamination from ABS plastic, scissors are easily recycled. However, there are wastes generated from the production of raw materials and transportation, which lead to negative environmental and economical impacts. While there are solutions and experiments conducted for reducing the wastes and pollutions, further researches and experiments are needed for more effective methods to remove the wastes and protect the ecosystems.
Bibliography
“ABS Plastic Production, Price and Market Demand.” Plastics Insight, 2019, https://www.plasticsinsight.com/resin-intelligence/resin-prices/abs- plastic/.
Buzz, Chatter. “Ore Conservation is a Major Benefit When Scrap Metal Gets Recycled.” GLE Scrap Metal, 2019, https://glescrap.com/blog/ore-conservation-major-benefit-scrap-metal-gets-recycled/
Daniel, Engber. “How Gasoline Becomes CO2.” The Slate Group LLC, Nov. 2006, https://slate.com/news-and-politics/2006/11/how-does-one-gallon-produce-19-pounds-of-carbon-dioxide.html
Department of Environment. “Iron”. ITP Mining: Energy and Environmental Profile of the U.S.
Mining Industry: Chapter 4: Iron, Energy and Environmental Profile of the U.S. Mining Industry, Nov. 2013, pp.4-1 - 4-13.
Eva, Blixt. “ Steel Production Residues.” Jernkontoret, Nov. 2018, https://www.jernkontoret.se/en/the-steel-industry/production-utilisation-recycling/steel-production-residues/
Goyal, Shikha. “What Is the Difference between Steel and Stainless Steel?” Jagranjosh.com, 8 Feb. 2018, https://www.jagranjosh.com/general-knowledge/what-is-the-difference-between-steel-and-stainless-steel-1518080822-1.
Honeywell UOP. “Honeywell Green Jet Fuel – Advanced Renewable Fuel Alternative to Traditional Jet Fuel.” Honeywell Green Jet Fuel™, Honeywell International Inc, 2019, https://www.uop.com/processing-solutions/renewables/green-jet-fuel/
Hosch, L. William. “Acrylonitrile-Butadiene-Styrene Copolymer: CHEMICAL COMPOUND.” Encyclopædia Britannica, Inc., Aug. 2009, https://www.britannica.com/science/acrylonitrile-butadiene-styrene-copolymer
“How Much Fuel Does an International Plane Use for a Trip?” How Stuff Work, 2019, https://science.howstuffworks.com/transport/flight/modern/question192.htm
Mining Watch Canada. Potential Toxic Effects of Chromium, Chromite Mining and Ferrochrome Production: A Literature Review. Mines Alerte. May 2012.
Minnesota Pollution Control Agency. “Crude oil and unrefined petroleum wastes.” Minnesota Pollution Control Agency, Aug. 2016.
Nilima Chaturvedi, et al. “Iron Ore Mining, Waste Generation, Environmental Problems and Their Mitigation through Phytoremediation Technology.” International Journal of Science and Research Methodology, vol. 5, no. 1, Nov. 2016, pp. 398-420.
Parker, R. “Green Aero-Engines: Technology to Mitigate Aviation Impact on Environment.” Sage Journals, Jan. 2010, https://journals.sagepub.com/doi/10.1243/09544062JMES1515
P. J. Nolasco-Sobrinho, et al. “Characterisation of dusts and sludges generated during stainless steel production in Brazilian industries.” Ironmaking & Steelmaking Processes, Products and Applications, vol. 30, no. 1, 2003, pp. 11-17. Published online on 18 Jul. 2013. https://www.tandfonline.com/doi/abs/10.1179/030192303225009506
“Polymer Waste Management.” RUBBER AND PLASTICS TECHNOLOGY,ANNA UNIVERSITY,MIT CAMPUS,CHENNAI, Nov. 2007, http://polymerwastemanagement.blogspot.com/2007/11/abs-recycling.html
Rick, Leblang. “Airplane Recycling and Value Extraction.” The Balance Small Business, Sep. 2019, https://www.thebalancesmb.com/airplane-recycling-and-value-extraction-2877922
Tony, Rogers. “Everything You Need to Know About ABS Plastic.” Creative Mechanisms, Jul. 2015, https://www.creativemechanisms.com/blog/everything-you-need-to-know-about-abs-plastic
Unlü K et al. “Treatability of chromite ore processing waste by leaching.” Waste Management & Research, Jun. 2001, https://www.ncbi.nlm.nih.gov/pubmed/11699856
Yuexi, Zhou et al. “Comprehensive analysis of the toxic and refractory pollutants in acrylonitrile–butadiene–styrene resin manufacturing wastewater by gas chromatography spectrometry with a mass or flame ionization detector.” Journal of Chromatography A, vol. 1244, Jun. 2012, pp. 161-167, https://www.sciencedirect.com/science/article/abs/pii/S0021967312006656
Yuexi, Zhou et al. “Treatment of wastewater from acrylonitrile–butadiene–styrene (ABS) resin manufacturing by Fe0/GAC–ABFB” Chemical Engineering Jounal, vol. 200-202, Aug. 2012, pp. 10-17, https://www.sciencedirect.com/science/article/pii/S1385894712007413