Design Life-Cycle
assess.design.(don't)consume
Sophie Bolotin
DES 40A
Winter 2018
Raw Materials of Fireworks
Raw Materials of Fireworks
The life cycle of fireworks consists of many processes from the manufacturing of each piece of the explosive to the recycling of the dangerous, yet beautiful product. The raw materials of these volatile pieces of art began as bamboo tubes filled with sulfur and charcoal as early as the 13th century. The life cycle of fireworks has evolved a great deal since their creation. The amount of raw materials used in each step greatly increased when the use shifted from war strategies to the beautifully elaborate displays seen today. The addition of more raw materials in turn creates a more unpredictable product that is hazardous to living organisms because of the toxins these chemicals produce.
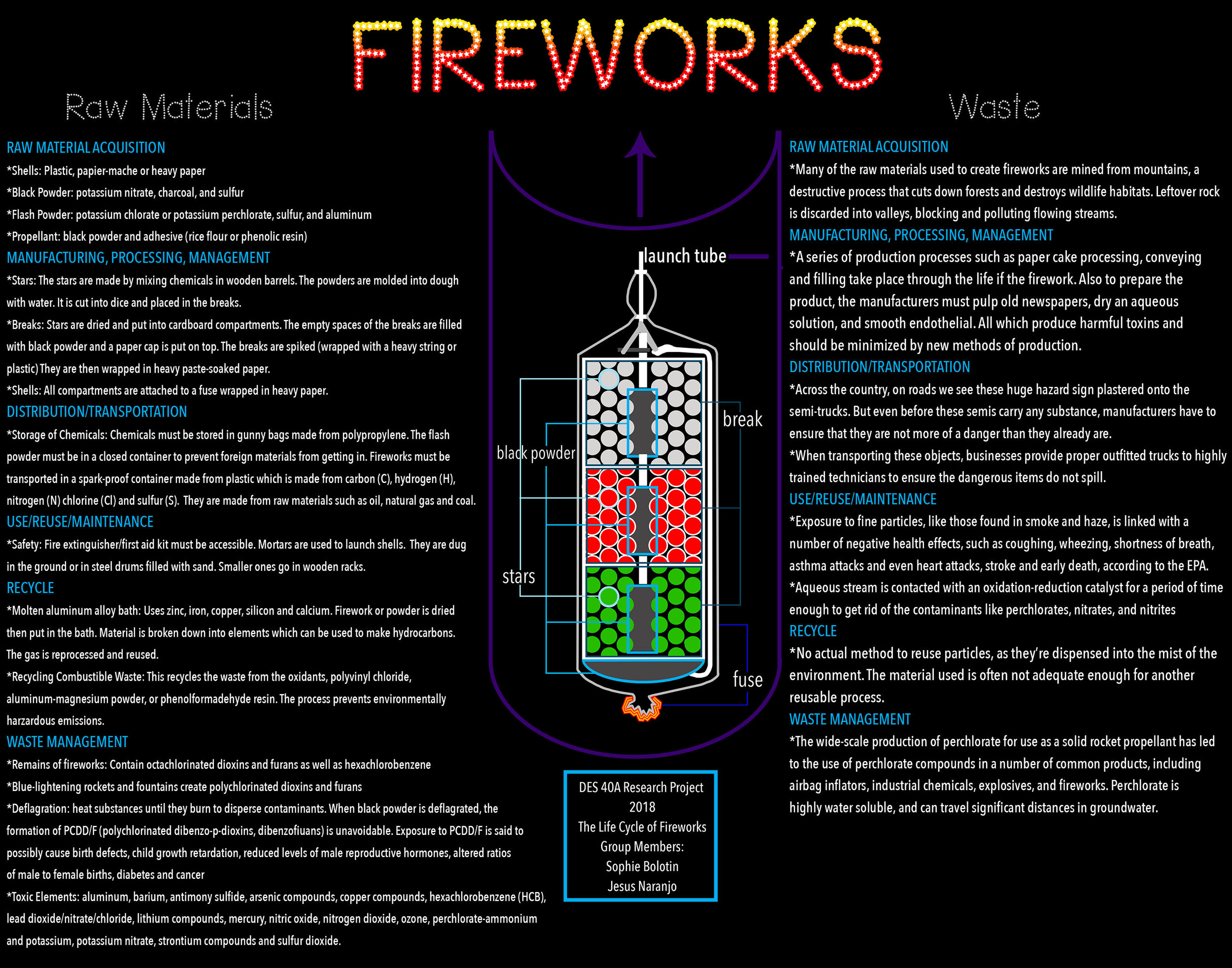
Fireworks consist of multiple parts, each using different raw materials to produce it. The outer part of the firework is called the shell which surrounds compartments inside the firework that hold the ammunition. This can be made from plastic, papier-mache or heavy paper. At the base of the shell, there is a small compartment that holds the black powder known as the propellant. The actual compartment is made from a mortar of usually iron, but also sometimes aluminum, plastic or heavy cardboard (“Fireworks”). Iron is a product of iron ore, coke, sinter, and limestone (“Iron”). Similar to iron, aluminum is made from bauxite ore and sodium hydroxide (“Aluminum”). The raw materials in plastic come from carbon, hydrogen, nitrogen, chlorine and sulfur which come from oil, natural gas and coal (“Polymerization”). The powder itself comes from the combination of salt-peter, which is potassium nitrate, charcoal, and sulfur in a 75-15-10 ratio by weight (“Fireworks”). In some cases, potassium hydrogen terephthalate is used in the creation of black powder (Changhu). An alternative powder to black powder is called flash powder. Flash powder is widely used in commercial fireworks because it produces a loud sound. Some military explosives use flash powder if they need to create large amounts of noise and light. The raw materials in this powder are potassium chlorate or potassium perchlorate mixed with sulfur and aluminum (“Fireworks”). The larger compartment in the shell has chunks of a mixture of chemicals which are called stars. Stars contain a fuel that burns to provide heat allowing for the firework to ignite, a coloring agent that delivers color when heated, and an oxidizer that burns the fuel. The fuel can be slow-burning such as charcoal, dextrin, or red gum to create a faint, continuous display, or fast-burning, such as aluminum, magnesium, or titanium, to produce a vivid, temporary display. Sugar can also be used as a fuel to produce smoke. Coloring agents can be aluminum, magnesium or titanium for white, carbon or iron for orange, sodium compounds for yellow, copper compounds for blue, strontium carbonate for red, and barium nitrate or barium chlorate for green. Oxidizers can be potassium perchlorate, which is the same ingredient in the flash powder, or ammonium perchlorate. They also contain chlorine, which reacts with copper, strontium, and barium compounds from the coloring agents to produce unstable chlorides which is what provide the color. The last component of the firework is the propellant which consists of black powder and an adhesive. The adhesive is normally glutinous rice flour or phenolic resin (“Fireworks”). Each of these compartments are attached to fuses made of threads that are mixed with grains of gunpowder. Each component of the firework is made from different raw materials, some of which are hazardous and others not as much. Depending on how flashy and extravagant the show is, the more raw materials and chemicals used to make it brilliant.
In the manufacturing process of fireworks, each component has different raw materials that go into the production. One process is making the stars which are the chunks of chemicals in the large compartment. The first step to making them is to scoop each chemical out of a barrel and mix them together. The barrels are normally made of wood. Brass is used to sift the powdered chemicals together. Then, the mixed powders are transported in the wooden barrels to the cutting room. The powder is transformed into a dough with the addition of water which is packed into a mold with a wooden mallet. The dough is then cut into dice on a workbench covered with cardboard and black powder. Once they are dry, they are put in cardboard containers. The empty spaces between the stars in the containers are filled with black powder. A paper cap is put on top, once filled and this is known as the break—the upper portion of the shell that create the color effect. The next step is called spiking which is the process of wrapping the break with a heavy string. However, some breaks are not spiked, but rather covered with plastic or heavy cardboard. Once spiked, they are wrapped again in a heavy paste-soaked paper then left to dry. Paste is normally made from the same materials as clay which are silica and alumina which makes aluminum silicate. Finally, the shells are made. This is done by connecting all the components together and attaching a starting fuse wrapped in heavy paper (“Fireworks”). Throughout the manufacturing process, wood is a major raw material used.
While some fireworks are commercially produced for companies and entertainment, fireworks for private use are also manufactured. These are normally smaller fireworks with less explosive materials. Some examples include, firecrackers which are paper tubes with a small amount of powder, fountains which are paper cones with chemicals that release colored sparks, and roman candles which are paper tubes with explosive materials and small stars. A trend in the raw materials of these small fireworks is the use of paper which comes from wood. Some fireworks contain no explosive material and are single chemicals wrapped in paper or foil. Examples of these include smoke balls, snakes which are filled with ammonium dichromate that produce long trails of ash, sparklers which are dipped metal wires in a slurry that contains fuel, an oxidizer, coloring agent and aluminum granules to produce a small spark (“Fireworks”). Sparklers are the most popular small firework used on celebratory holidays such as New Year’s Eve or Fourth of July.
The transportation of fireworks is a very delicate and important task that requires intensive training, valid licenses and specific storage materials. These requisites are put into place to ensure that fireworks are safely transported. Usually professional fireworks are launched by the same companies that produce them which makes it easier for transportation. The first component of transportation is the storage of the chemicals. When loading and unloading the chemicals used to manufacture the explosives, the transporter must address certain important things. The chemicals are enclosed in gunny bags and not dragged across the floor, because friction can cause it to ignite (Sivapirakasam). Gunny bags are historically made of burlap which is formed from jute, hemp, or other natural fibers. Most gunny bags produced today come from polypropylene (“Gunny Sack”). The next thing to remember is that aluminum powder cannot be rolled or dropped for the same reason. The firework box also cannot be dragged and should be properly lifted and carried to storage for transportation due to its instability. When using flash powder, the pyrotechnic mixture must be in a closed container to prevent entry of foreign material or water; the container is normally plastic (Sivapirakasam). When transporting the fireworks in a truck, they must be in a spark-proof container, usually plastic (“Tips for getting fireworks”). The majority of accidents occur because precautions are not taken and materials are not carefully handled. This is why transporters must have valid licenses which can be obtained through an online or paper application.
When fireworks are launched, safety is the most important factor; similar to transportation, certain precautions must be taken to ensure the fireworks are launched safely and correctly. A fire extinguisher and first aid kit must be accessible. The mortars needed to launch the shells are placed in their proper places. The larger ones are put in holes dug in the ground or in steel drums filled with sand, while the smaller ones go in wooden racks (“Fireworks”). Therefore, the process of launching needs minimal extra raw materials. However, it is when the fireworks explode prematurely and emit their waste that more raw materials are used.
There are many different inventions that have been created to recycle the materials used in fireworks in an efficient manner. One process uses molten aluminum or a molten aluminum alloy bath. The aluminum is alloyed with metals such as zinc, iron, copper, silicon and calcium. The material, normally the firework itself or the black powder, is ground and dried and put into the alloy bath below the surface. The ground material is forced below the surface with an inert gas such as nitrogen or argon. As the ground material passes through the bath, the aluminum reacts to break down the material into elements which are then removed. The elements in the material range from carbon, sulfur, hydrogen, nitrogen, mercury, copper, or iron depending on what the firework is made from. These elements can be made into hydrocarbons and sold or burned to finish the recycling and waste management process. In addition, the inert gas is reprocessed and reused (Presswood). Another process focuses on the recycling of combustible waste. Fireworks contain a “color-flame oxidant [usually] barium nitrate or strontium nitrate, polyvinyl chloride, aluminum-magnesium powder, phenolformadehyde resin and as recyclable pyroxlin- containing wastes, combustible case material which contains trinitrotoluene, pyroxylin and cellulose” (Grishin). This process recycles the combustible waste from these materials which prevents environmentally hazardous emissions during combustion. It improved the intended properties for flame saturation with certain colors because the rate of combustion is extremely high and there are certain outputs for the different desired firework products (Grishin). Overall, inventions are being developed to recycle the materials used in fireworks instead of wasting them.
Different aspects of the fireworks produce different types of waste; this led to the creation of numerous methods to manage these emissions. The remains of fireworks contain octachlorinated dioxins and furans, as well as hexachlorobenzene. When the substances containing explosive materials are heated until they burn away—a process called deflagration—the result is a “dispersion of contaminants.” In contrast, compositions of “continuously burning flares compositions [only] partially lead to a thermal decomposition of organic pollutants” which means the materials are chemically broken down into natural contaminants (Fleischer). In addition, when setting off blue-lightening rockets and fountains, polychlorinated dioxins and furans are formed. One problem of waste management of fireworks is that even at high temperatures, when black powder is deflagrated, the formation of PCDD/F (polychlorinated dibenzo-p-dioxins, dibenzofiuans) is unescapable. (Fleischer). Exposure to PCDD/F is said to possibly cause birth defects, child growth retardation, reduced levels of male reproductive hormones, altered ratios of male to female births, diabetes and cancer (Rizal). Certain routines must be followed for public use of fireworks. If disposing of the explosives in curbside garbage containers, the fireworks must be soaked in water and placed in plastic bags. First a 5-gallon plastic bucket must be filled with water and the fireworks are placed inside to soak. The wicks must be removed before soaking. Since some portions of the fireworks are lighter than others, a weight is used to keep it submerged in the water. After they are soaked for 24 hours, the water is drained and the firework remains are placed in a plastic bag for the trash (“Ammunitions”). A general trend for the public disposal of fireworks is the use of plastic which comes from raw materials stated above. The black powder must be rendered in a specific device. In an experiment, energy densities of different types of gunpowder were tested resulting in the conclusion that it is impossible to use the waste from gunpowder to weld common metals. This is important because it proves that gunpowder is not easily recyclable. However, when comparing aluminum powder to thermite, it was observed that thermite can elevate the energy density of metals more, which means it has a better chance of being used for welding. In fireworks specially, aluminum powder is normally used (“Study on Energy Density”). All fireworks produce smoke and dust which can sometimes be problematic. Some of the smoke and dust can contain heavy metals, sulfur-coal compounds and other poisonous chemicals. Almost all of the metals produce dust that has toxic effects. In a firework show, aluminum is used for a white color, but the byproducts can cause contact dermatitis. Barium nitrate is used for a green color which is poisonous and gives off fumes that irritate breathing. Other elements include antimony sulfide, arsenic compounds, copper compounds, hexachlorobenzene (HCB), lead dioxide/nitrate/chloride, lithium compounds, mercury, nitric oxide, nitrogen dioxide, ozone, perchlorate- ammonium and potassium, potassium nitrate, strontium compounds and sulfur dioxide. Almost all of these elements produced from and used in firework production are extremely toxic and lead to a variety of environmental problems (“The Environmental Effects of Fireworks”). As of now there is no research on how to fix this problem, but in the future, if people want to continue to enjoy light shows and commercially produced advertisements, there needs to be a way to manage these toxins or use alternatives to avoid the dangerous effects overall.
The raw materials that go into each step of the life cycle has generally increased since the development of fireworks. More raw materials have, in turn, caused more chemicals to be used and therefore, emit more waste into the environment. For example, in China, fireworks evolved from simple firecrackers to lavish displays with various colors and numerous chemicals. In Europe, fireworks began as military explosives, then were used for celebrating victories and later progressed to the elaborate productions. Overall, the purpose of fireworks today is to entertain. As new technologies are being developed and society is advancing, many new colors and levels of sound are evolving. This in turn, is increasing the use of raw materials in the production and management of these extravagant explosives.
Bibliography
“Aluminum.” How Products Are Made, Advameg, Inc., 2018,
http://www.madehow.com/Volume-5/Aluminum.html
“Ammunitions, Explosives, Flares & Fireworks.” Metro Waste Authority,
www.mwatoday.com/recycling-guide/item/ammunitions-explosives--flares.aspx
Anatomy of a Firework. (2002). [image] Available at:
http://www.pbs.org/wgbh/nova/fireworks/anat_nf.html [Accessed 11 Mar. 2018]
Changhu, Zhong. Liling Hengda Fireworks Co. "Propellant For Fireworks And Crackers." 2013:
n. pag. Print.
“Fireworks.” How Products Are Made, Advameg, Inc., 2018,
www.madehow.com/Volume-2/Fireworks.html.
Fleischer, O, H Wichmann, and W Lorenz. "Release Of Polychlorinated Dibenzo-P-Dioxins And
Dibenzofurans By Setting Off Fireworks." Chemosphere 39.6 (1999): 925-932. Web.
Grishin, Andrei. Open Joint-Stock Company Cheboksary Production Association. "Pyrotechnic
Composition For Fireworks." 2012: n. pag. Print.
“Gunny sack.” Wikipedia, Wikimedia Foundation, 17 Feb. 2018,
en.wikipedia.org/wiki/Gunny_sack
“Iron.” How Products Are Made, Advameg, Inc., 2018,
http://www.madehow.com/Volume-2/Iron.html
Presswood Jr., Ronald, and Ian Bishop. "Method To Recycle Plastics, Electronics, Munitions Or
Propellants Using A Metal Reactant Alloy Composition." 2016: n. pag. Print.
“Polymerization: How Plastic Materials are Made - Craftech Industries - High-Performance
Plastics - (518) 828-5001.” Craftech Industries, 7 Apr. 2017, www.craftechind.com/polymerization-how-plastic-materials-are-made/
Rizal Razman, M. and Azlan, A. (2009). Safety issues related to polychlorinated
dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) in fish and
shellfish in relation with current Malaysian laws. Journal of Food, Agriculture & Environment.
Sivapirakasam, S.P., M. Surianarayanan, and G. Swaminathan. "Hazard Assessment For The
Safe Storage, Manufacturing And Handling Of Flash Compositions." Journal of Loss
Prevention in the Process Industries 22.2 (2009): 254-256. Web.
“Study on the Energy Density of Gunpowder Heat Source .” Study on the energy density of
gunpowder heat source - IEEE Conference Publication, ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=5930549
Sultech Inc. "Process For The Destruction Of Explosives." 1994: n. pag. Print.
“Tips for getting fireworks safely to their destination.” Tips For Getting Fireworks Safely to
Their Destination - Consumer Reports News, www.consumerreports.org/cro/news/2014/07/tips-for-getting-fireworks-safely-to-their- destination/index.htm
“The Environmental Effects of Fireworks.” Energy Saving Advice | Energy Saving Information |
Energy Saving Tips, 23 Aug. 2013, www.energysavingwarehouse.co.uk/learning-
portal/the-environmental-effects-of-fireworks/.
Jesus Naranjo
C. Cogdell
DES 40A & A03
15 March 2018
The Emissions of Fireworks
It has been a tradition to dispense millions of fireworks during the fourth of July in honor of our independence and America's new beginning. Fireworks date as far back as the seventh century in Medieval China to celebrate its traditions and scare away the evil spirits. Over the centuries, countries have adapted to hold festivities with the fireworks being present. Like in the Philippines where they celebrated the new year with countless fireworks and setting a record for the most fireworks lit up at a time. Regardless of these traditions and symbols, society has been affected tremendously by the number of waste emitted in the atmosphere. It is in the best of interest that among our nation, other’s as well seek to pursue a safer alternative, to construct these fireworks with fewer toxins.
The process of the creation of fireworks is unique for each specific one, as most include different materials and chemicals to create a new wonder of the color of potential strongness. Nevertheless, the end goal has always been kept as to create a non-detonating, solid, and resistant product to face among different conditions. Other pyrotechnic composites also use this rule as most have chemicals that are combustible and can create a hazard instantaneously. The matter of how these fireworks take form, all depend on chemicals and colorants that industries attain, as the process to attain these have hurt our environment by excavating and wiping out natural resources, hurting wildlife, and polluting lakes. Not only do our four-legged friends hide on the more patriotic night of America, but the waste that goes in the air is toxic enough to kill in large sums. Among the other pyrotechnic compositions, fireworks include a process which lists the mass percentage of the raw materials used in production: twenty three to twenty seven percent of fly ash, twenty three to twenty seven percent of calcium carbonate, twenty three to twenty seven percent of waste paper pulp, half to one and one half percent of calcium stearate, four to six percent of gypsum powder, three to five percent of starch, and fourteen to sixteen percent of water (Change C).
There has been a break in the success of the process of making, it almost seems like the production and innovation have sat on a plateau. A series of production processes such as paper cake processing, conveying and filling take place through the life of the firework. Also to prepare the product, the manufacturers must pulp old newspapers, dry an aqueous solution that of water-soluble, that is to make the product fire-retardant and water repellent. So there’s a lot that occurs during the production of fireworks and that’s not to mention the heat press work and sand smoothing of the thin layers to the surface area. All of the process which produces a safer alternative for production rather than the old and more harmful mode of production. The old way was simply using machines at a distinct angle rather than parallel and wasting most of the material being used and producing more toxins (Li You'an). The new mode of production has now been implemented in new industries showing its capability and its advancement. Li You’ a states that the objective is to provide an inexpensive and mass producing machine, that is effective and stays away from prevents problems caused by hardness and strength or the outer surface of the same starburst of drug release without a fire or burst movement.
Across the country, on roads we see these huge hazard sign plastered onto the semi trucks, and to some, it may be a frightening sign. But even before these commercial trucks can carry any sort of substance, manufacturers have to ensure that product does lie as a threat on the road more than what it already is by itself. Regulations have been established since ages to ensure not only the proper displacement of them but the distribution as well. When transporting these objects, businesses provide properly outfitted trucks to highly trained technicians to ensure the dangerous items do not deploy or spill. According to the industry of Stericycle, they use their own specialized vehicles to make even more sure that they’re the ones to ensure the safety of those on the road and their employees. This is only one sort of industry which deploys hazardous items on the road to be transferred to local retail or other sale venues and try to look out for the safety of the world. Yet, the transportation of fireworks could also be seen to take the same route. If perchance an accident were to occur while on road, either because of the manufacturer or the carelessness of the distributor, one could see explosions, thus letting toxins out in the air. But without regarding those hypotheticals, there really isn't much waste in the process that fireworks are transported other than the emissions of the vehicle hauling or carrying the merchandise.
Exposure to small and fine particles, like those found in smoke and haze, are linked with a number of negative health effects along with the pollution of the environment. A few of those health effects are coughing, wheezing, shortness of breath, asthma attacks, and heart attacks according to the EPA (Fireworks Cause Spike). If the toxic wastes are already polluting our water the endless amounts of particles existent in the sky will soon also impact our breathing as well. Like previously stated, each chemical compound used inside the firework causes a unique usage like the colors they display, nonetheless, the basic toxins are heavy metal fallout, dioxin pollution, acid rain, airborne lead, ozone formation. Also, the display of the certain particular toxic elements has a profound list of effects individually. For example, the two chemical elements barium and nitrogen create this unique glittering green. The effects are poisonous fumes which can lead to respiratory tracts and possible radioactive fallout. Again, this is only one example of the many endless possibilities of conjunctions of chemicals, some may lead to deadlier effects or maybe less but nevertheless produce toxins which affect one’s way of living. Yet, there has been one discovery made in which destroys contaminants in a contaminant-containing aqueous stream and one of a group of contaminants including perchlorates, nitrates, and nitrites. As crazy as it sounds, it works so long as the contaminant-containing aqueous stream is heated then the aqueous stream is contacted with an oxidation-reduction catalyst for a period of time enough to get rid of the contaminants like perchlorates, nitrates, and nitrites (Process For Destroying Contaminants). “On a local level, it increases in fine particulate matter varied depending on a number of factors, including the weather and the proximity of fireworks to the monitoring site.” (Change C). At one site in Utah, fireworks were set off near an air-quality monitoring site for the holiday, particular matter concentrations rose three hundred seventy percent well above the EPA (Environmental Protection Agency) standard. This only shows to prove that on the instance that day that the matter concentration was recorded, certain factors contributed to the number calculated. While perchance, if it were recorded in California near the shore a lower concentration may have been recorded as nature effects the number.
The rates at which fireworks have contaminated the air are creating societal problems at large within the nation. Wide-scale production of perchlorate for use as a solid rocket propellant has led to the use of perchlorate compounds in a number of common products, including airbag inflators, industrial chemicals, explosives, and fireworks. Perchlorate is highly water-soluble and can travel significant distances in groundwater. While also another potential deadly chemical admixture is the simple element aluminum. This element is easily seen in one’s household, but when ignited with other chemicals, the toxic effects are a contact with dermatitis and bioaccumulation. Which in other words dermatitis is a disease that displays rashes and scaly itching skin like eczema and bioaccumulation is the ingestion of pesticides. Thus, manufacturers and consumers should be wary of the effects that the products may have and should take the time to dispense them properly. Pyrotechnics like ammunition, fireworks, and explosives are regulated as wastes in Minnesota when they cannot be used for their intended purpose (Ammunition, Fireworks, and Explosive Waste). There is no actual method to reuse particles, as they’re dispensed into the mist of the environment. The material used is often not adequate enough for another reusable process.
Although industries are doing a good job at producing these fireworks at low costs, the end outcomes of the products release deadly poisons that hurt the environment and society. In many parts of the world, individuals freely ignite fireworks as a meaningful symbol or even as a tradition. Fireworks may be used around the world for different cultural traditions such as religion, attraction and holidays, but regardless of the reason, one should just keep in mind the consequences it has over mankind. It has been seen that humankind always wants the best for the world and oneself, all it might require is effort and teamwork to preserve our world to all work together and not let emissions of fireworks take over.
Bibliography
Change, C., Matters, C., Explained, C., work?, H., WRCs®?, W., Business, F., Sustainability, C., Clients, t., Products, G., Aviation, t., Footprint, C., Individuals, F., Living, S., Footprint, C., List, P., Info, P., Us, A., Story, O. and Principles, O. (2018). Fireworks: Their Impact on the Environment - terrapass. [online] terrapass. Available at: https://www.terrapass.com/fireworks-impact-environment [Accessed 8 Feb. 2018].
China North Chemical Industry Corporation (2003). Smokeless gun propellant for fireworks and its prepn. CN1526687A.
Conway, C. (2018). 5 Ways Fireworks Ruin Our Natural Resources. [online] Backcountryattitude.com. Available at: http://www.backcountryattitude.com/fireworks-impact-nature.html [Accessed 8 Feb. 2018].
Fleischer, O., Wichmann, H. and Lorenz, W. (2018). Release of polychlorinated dibenzo-p-dioxins and dibenzofurans by setting off fireworks.
Li You'an, L. (2014). Full-automatic filler for combined firework paper cakes. CN201922481U.
Mclendon, R. (2018). Are fireworks bad for the environment?. [online] MNN - Mother Nature Network. Available at: https://www.mnn.com/earth-matters/translating-uncle-sam/stories/are-fireworks-bad-for-the-environment [Accessed 8 Feb. 2018].
Open Joint-Stock Company Cheboksary Production Association named after VI Chapayev (2012). Pyrotechnic composition for fireworks. RU2501777C1.
Pca.state.mn.us. (2018). [online] Available at: https://www.pca.state.mn.us/sites/default/files/w-hw4-04.pdf [Accessed 7 Feb. 2018].
Science, L. and Earth, P. (2018). Fourth of July Downer: Fireworks Cause Spike in Air Pollution. [online] Live Science. Available at: https://www.livescience.com/51408-july-4-air-pollution-fireworks.html [Accessed 8 Feb. 2018].
Sivapirakasam, S., Surianarayanan, M. and Swaminathan, G. (2018). Hazard assessment for the safe storage, manufacturing and handling of flash compositions.
State, K. (2018). Fireworks raw material formulation and preparation method. CN107540323A.
Stericycle Environmental Solutions. (2018). Hazardous Waste Transportation Doesn't Have to Be Risky. [online] Available at: https://www.stericycleenvironmental.com/hazardous-waste-transportation-risky/ [Accessed 15 Mar. 2018].
Sultech Inc (1994). Process for the destruction of explosives. US5434336A.
Sydneymedia.com.au. (2018). Sydney New Years Eve: making a mark without lasting impressions on the environment - The City of Sydney Media Centre. [online] Available at: http://www.sydneymedia.com.au/4467-sydney-new-years-eve-making-a-mark-without-lasting-impressions-on-the-environment/ [Accessed 8 Feb. 2018].
The Conversation. (2018). Our prettiest pollutant: just how bad are fireworks for the environment?. [online] Available at: https://theconversation.com/our-prettiest-pollutant-just-how-bad-are-fireworks-for-the-environment-52451 [Accessed 8 Feb. 2018].
Umpqua Research Co (1998). Process for destroying contaminants in contaminant-containing aqueous streams and catalysts used therefor. US6419837B1.